| Ursolic Acid Attenuates Oxidative Stress-mediated Hepatocellular Carcinoma Induction by Diethylnitrosamine in Male Wistar Rats |
| 发布时间:2012-09-25 信息来源:admin 发布人:admin 点击次数:4192 |
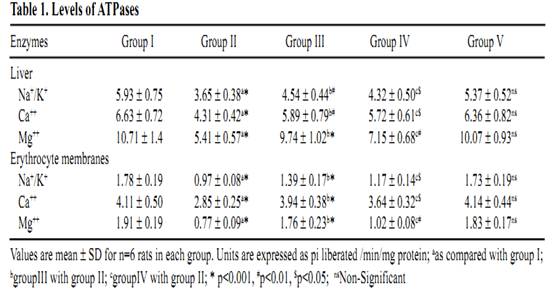
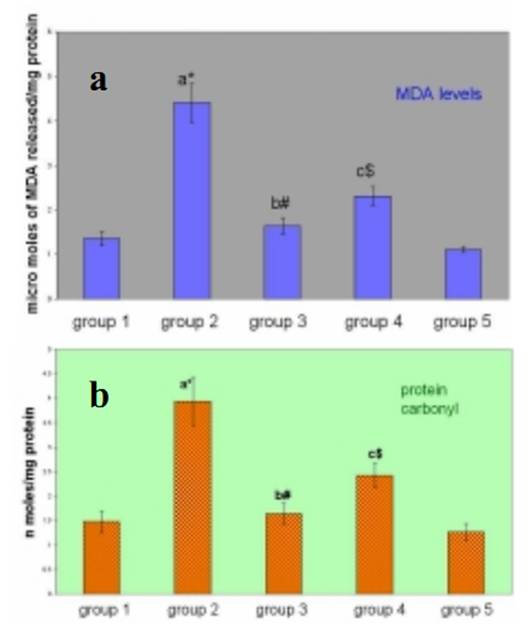
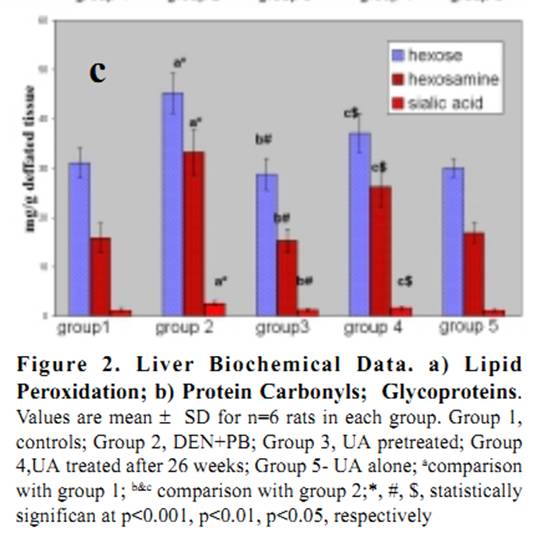
Ursolic Acid Attenuates Oxidative Stress-mediated Hepatocellular Carcinoma Induction by Diethylnitrosamine in Male Wistar Rats Abstract Hepatocellular carcinoma is the most common primary cancer of the liver in Asian countries. For more than a decade natural dietary agents including fruits, vegetables and spices have drawn a great deal of attention in the prevention of diseases, preferably cancer. Ursolic acid is a natural triterpenoid widely found in food, medicinal herbs, apple peel and other products it has been extensively studied for its anticancer and antioxidant properties. The purpose of this study was to evaluate the effect of ursolic acid in diethylnitrosamine (DEN) induced and phenobarbital promoted hepatocarcinogenesis in male Wistar rats. Antioxidant status was assessed by alterations in level of lipid peroxides and protein carbonyls. Damage to plasma membrane was assessed by levels of membrane and tissue ATPases. Liver tissue was homogenized and utilized for estimation of lipid peroxides, protein carbonyls and glycoproteins. Anticoagulated blood was utilized for erythrocyte membrane isolation. Oral administration of UA 20 mg/kg body weight for 6 weeks decreased the levels of lipid peroxides and protein carbonyls at a significance of p<0.05. Activities of membrane and tissue ATPases returned to normal after UA administration. Levels of glycoproteins were also restored after treatment. Histopathological observations were recorded. The findings from the above study suggest the effectiveness of UA in reducing the oxidative stress mediated changes in liver of rats. Since UA has been found to be a potent antioxidant, it can be suggested as an excellent chemopreventive agent in overcoming diseases like cancer which are mediated by free radicals. Key Words: Glycoproteins - lipid peroxidation - membrane ATPases - protein carbonyl - Rosmarinus officianalis Introduction Hepatocellular carcinoma is a major malignancy world wide (Lodato et al., 2006). It is the fourth most common malignant tumor in the world (Lee et al., 2005) and is the second leading fatal disease in mainland China El-Serrag (2004). Chemical hepatocarcinogenesis is a favourite model in rat, to study the mechanism of biotransformation of a normal cell to malignant populations. Carcinogenesis is a multistep and multistage process that begins with irreversible, but heritable damage to a single cell Dragon (1994). Model systems in animals exhibit this property of cancer development in several organ systems Pitot (1986). Rat liver is one of the most extensively studied models of carcinogenesis and multiple formats have been described for the analysis of cancer development (Goldsworthy et al., 1985). Cell proliferation appears to play a crucial and critical role in several steps in cancer development. Diethylnitrosamine (DEN) is a representative chemical of a family of carcinogenic N- nitroso compounds and isnormally used as a carcinogen to induce cancer in animal models (Bhosale et al., 2002). Diethylnitrosamine is an environmental carcinogen and hepatotoxin. It causes degenerative, proliferative and neoplastic lesions in liver (Qi et al., 2008). DEN is a chemical agent that can alkylate DNA molecule with itself being converted to highly reactive molecule by P-450 dependent oxygenases (Li, 2005). NDEA has been found in a variety of products that would result in human exposure, including main stream tobacco smoke. DEN is bioactivated by P-450 mediated _-hydroxylation producing _-hydroxyl nitrosamine. It is hydroxylated principally by ethanol inducible CYP2E1 in liver. DNA-adduct formation proceeds through an ethyldiazonium ion intermediate and evolution of N2 (Michejda et al., 1982; Singer and Grunberg, 1983). DEN administration influences cytotoxicity, cell proliferation and DNA replication rates (Verna et al., 1996). When administered in drinking water, N-nitrosodiethylamine induced liver tumors in guinea pigs, rabbits, dogs, and rats. Renganathan Gayathri et al The administration of such carcinogenic substances brings about changes in enzyme levels arising from clonic proliferation of cells (Kang et al., 1997). Phenobarbital is an anti-epileptic drug. It is a commonly employed non- genotoxic agent to induce liver tumor in lab animals Cunningham (1996). It promotes hepatocarcinogenesis when administered after an initiating dose of DEN (Loppen et al ., 2002). Studies on natural products are on rise not only because of their medicinal value, but also because of their lesser side effects. Triterpenoids are a class of naturally occurring compounds that are found in a variety of European plants & fruits. They are studied for their anti-inflammatory, hepatoprotective, analgesic, antimicrobial, antitumor, immunomodulatory and tonic effects. Ursolic acid (3ß hydoxy urs-12 en – 28 oic acid) is a triterpenoid found widespread, especially in higher plants like Rosmarinus officianalis. It is shown to lower COX-2 transcription (Subbaramaiah et al., 2000). It has been studied for its beneficial effects on liver in CCl4 toxicity model (Martin- Aragon et al., 2001) and antihepatoma activity in mice. It has also been reported that UA has potent antiproliferative activity against HepG2 human liver cancer cell line (Zen et al., 2006). The current study has been focused to understand the antioxidant effect of UA in DEN induced hepatocarcinogenesis model in male Wistar rats. Lipid peroxidation and protein carbonyl in liver tissues were studied to assess the free radical mediated oxidative stressand glycoprotein and membrane ATPases were estimated to reflect the membrane damage of the organ. Materials and Methods Chemicals Diethylnitrosamine (DEN), phenobarbital and ursolic acid (UA) were procured from Sigma Chemical Company, St. Louis, MO, USA. All other chemicals used for the experiments were of analytical grade. Animals Male Wistar albino rats weighing approximately 100-150 g were divided into five groups of 6 rats each. All rats were maintained in Polypropylene cages with 12 h light/dark cycle under constant temperature and humidity. Animals were fed a commercial diet and had ad libitum access to food and water. The research was approved bythe institutional animal ethical committee (IAEC NO.02/078/07). Experimental design Group 1: Control animals given vehicle alone (0.2% gum acacia for 34 weeks). Group 2: Animals induced with DEN by a single intraperitoneal injection (200mg/kg b.wt in saline). After 2 weeks recovery period, the carcinogenic effect was promoted by Phenobarbital at 0.6% finely ground with 0.2% gum acacia and was given in drinking water for 26 weeks. Group 3: Animals were treated with Ursolic Acid at the dosage of 20mg/kg b.wt in 0.2% gum acacia per day for 15 days before administration of DEN as in Group 2. Group 4: Animals were treated with Ursolic acid at a dosage similar to Group 3 for 6 weeks after 26 weeks (induction period). Group 5: Animals were treated with Ursolic acid alone for 6 weeks at the above mentioned dosage to observe the toxicity present (if any) for Ursolic acid. Protocols After the experimental period, the animals were killed by cervical decapitation. Blood and liver tissues were collected and washed in ice cold saline (0.89%). The tissues were then blotted to dryness and 10% homogenate was prepared immediately using Tris-HCl buffer 0.1M (pH-7.4) using a Potter Elvejhem glass homogenizer and the homogenate was used for estimation of lipid peroxides (Ohkawa et al., 1979) & protein carbonyls (Levine et al.,1990). The blood sample collected with 5% EDTA was used to isolate erythrocyte membrane according to the method of Dodge et al., (1963) with slight modifications of Quist, 1980. Glycoproteins from the liver tissue were extracted with chloroform and methanol. The extract was hydrolysed with acid and finally used for the estimation of hexose, after Neibes (1972), hexosamine, after Wagner (1979), and sialic acid, after Warren (1959). Na+K+ ATPase (E C 3.6.1.3) were estimated by the method of Bonting (1970). The activity of Ca++ ATPase (E C 3.6.1.3) was assayed by the method of Hjerten and Pan (1983). The activity of Mg++ ATPase ( E C 3.6.1.3) was assayed by the method of Ohnishi et al (1982). Inorganic phosphorus was estimated by the method of Fiske and Subba Row (1925). Protein content was estimated by Lowry et al (1951). Statistical Analysis The data were expressed as mean ± standard deviation. Significant changes between the groups were detected by one way analysis of variance (ANOVA) and least significant difference (LSD) method was used to compare the means of different groups. Commercial software SPSS version 10.0 was used for statistical analysis. Histopathology The liver samples of DEN treated animals showed hyperchromatism, hyperplasia, and proliferating hepatocytes (see Figure 1). On treatment with Ursolic acid the architecture of the liver was restored showing regeneration of normal hepatocytes. Antioxidant status There was a significant increase in the levels of lipid peroxidation in cancer bearing animals (group 2) when compared to control animals (group1) with a significance of p<0.001 (see Figure 2a). Levels were decreased in UA treated animals (group 4) to approximately 52%. In pretreated animals (group 3), the levels decreased as compared to cancer bearing animals & was closer to control animals, due to the continuous administration of UA. No significant change was seen in UA alone treated animals (group 5). Regarding levels of protein carbonyls formed during carcinogen and UA treatment, there was a significant increase in cancer bearing animals (group 2), which was reduced by 61% in UA treated animals (group 4)(see Figure 2b). Pretreated animals (group 3) also showed a significant decrease, when compared to cancer bearing animals. There were no marked changes in UA treated animals (group 5). Membrane damage Glycoproteins (hexose, hexosamine & sialic acid) were increased significantly in cancer bearing animals (group 2) with a significance of p<0.001, which decreased on treatment with UA (see Figure 2c). The results were expressed at significance of p<0.05. Table 1 shows change in the levels of ATPases in liver and erythrocyte membranes of male Wistar rats. The enzyme activities of Na+K+ and Mg++ ATPases were significantly decreased in liver & erythrocyte membrane (p<0.001) in carcinogen treated animals (group 2). The levels were restored in UA treated (group 3) and (group 4) animals (p<0.01 & p<0.05) respectively. Decrease in Ca++ ATPase activities was observed in the erythrocyte membrane of cancer bearing animals (group 2) and increased significantly in group 3 and group 4 animals. Discussion Lipid peroxidation is the most extensively studied free- radical mediated process. Free radicals can be defined as molecules or molecular fragments containing one or more unpaired electrons. The presence of unpaired electrons usually confers a considerable degree of reactivity on a free radical. These free radicals derived from oxygen represent the most important class of such species generated in living systems (Valko et al., 2004). Oxidative stress is discussed as a possible cause of hepatocarcinogenesis in rodents and is suggested to result from excessive production of H2O2 from degradation of fatty acid (Wolfgang et al., 1997). Lipid peroxidation has been shown to perturb the bilayer structure and modify membrane fluidity (Mandal, 1980; Chattrejee, 1988; Kunimoto, 1981). In recent years much research has been dedicated in identifying the plant components which contribute in combating the oxidative stress and free radical induced damage, which mainly is the first step to chemical carcinogenesis. In the current study, the focus was to assess the levels of oxidative stress markers, lipid peroxides and protein carbonyls in the liver of DEN induced male Wistar rats. DEN is the principal nitrosamine among environmental carcinogens in intercalating with membrane lipids for free radical formation (Nakae et al., 1997). These radicals cause damage to cellular processes, there by causing decrease of enzyme activities etc leading to cell death. This was evident from our current study too where group 2 cancer bearing animals showed a statistically significant increase in the levels of lipid peroxidation products. The reduced levels of lipid peroxides in group 3 and group 5 rats treated with Ursolic acid indicated the antioxidant property of this compound in reducing the lipid peroxides level henceforth preventing initiation and propagation process by scavenging free radicals and therefore its role in combating oxidative stress. Proteins are important targets of oxidative modifications. Protein carbonyl is a product of irreversible non enzymatic oxidation or carbonylation of protein and indicators of free radical generation in cells (Dalle-donne et al., 2006). Oxygen radicals generated as byproducts of cellular metabolism or from carcinogenic assault result in functional changes in structural & enzymatic proteins Stadtman (1992). The presence of carbonyl group has been used as a marker of reactive oxygen mediated protein oxidation (Berlett and Stadtman, 1997). There was a significant increase in the levels of protein carbonyls in DEN induced animals which was restored to lower levels on treatment with UA. The fact that UA has shown to decrease oxidative stress or MDA products by increasing the antioxidant enzyme status such as catalase (Kim etal., 1996) and thiol status (Martin-Aragon et al., 2001) of the cell might also be a reason for decreased protein carbonyl levels, since carbonyl groups in proteins are mainly introduced by malondialdehydes produced during lipid peroxidation. Glycoproteins are proteins that contain oligosaccharide chains (glycans) covalently attached to their polypeptide chain. Compositional analysis following acid hydrolysisis one method of identifying sugars, qualitatively and quantitatively. Sialic acid, one of the glycoprotein components is used as a tumor marker Crook (1993). They have a central role of functioning in biological systems such as stabilizing the conformation of glycoproteins on cellular membranes, assisting in cell-cell recognition, and interaction, and serving as chemical messengers in body fluids & tissues Kurtul (2004). Elevated levels have been reported in alcoholics Kurtul (2004) and (Ponnio et al., 1999) and smokers (Lindberg et al., 1991). In animal study, carcinogen administration tends to increase Sialic acid levels (Dich et al., 1997). Serum glycoproteins have shown to be elevated in lung, prostrate, bladder and GI system (Erbil et al., 1985) and (Kokoglu, 1992). In our current study, there was a significant increase in the levels of glycoproteins in cancer bearing animals (group 2) which suggests the harmful effects of DEN promoted by phenobarbital. We have observed a significant decrease in the levels of hexose, hexosamine & sialic acid in animals treated with UA suggesting the antitumor nature of the drug because the first reactions of organism to carcinogenic compounds take place at the cellular level, before the effect become visible at higher levels of biological organizations. Since the levels of glycoprotein were reduced in group 3 & group 5 animals, it may be attributed to the protective efficacy of the triterpenoid, UA. Liver plasma membrane plays a major role in hepatobiliary transport of biliary components and xenobiotics. There is a need for healthy signaling and message flow to maintain a good communication and coordination of biochemical process within the cell. Alterations in the plasma membrane of liver in its composition and fluidity can influence cancer mediated transport process and membrane bound enzyme activities (Ana et al., 1999). Injury to cell membrane by free radicals has been a recent focus since the vital activities of the cell are challenged. The three important ATPases of the plasma membrane are the Na+K+ATPase, Ca++ ATPase and Mg++ ATPase. Na+K+ATPase uses energy derived from the hydrolysis of ATP to keep a high K+ and a low Na+ concentration in the cytoplasm which in turn provides the driving force for the net movement of other substance such as Ca++, aminoacids and H+ (Contreras et al.,1999). Decrease in the activity of Na+K+ ATPase and Mg++ ATPase occurs during tumor growth particularly in malignancy. This is well correlated with the current study wherein a similar decrease in the activities were found in cancer bearing animals (group 2), which suggests the condition of malignancy and progression of cancer. The decreased activity might also be due to lipid peroxides induced by DEN which could have altered membrane structure. In the current study significant increase in the activities of these two enzymes in group 3 & group 5 animals suggest that decrease in levels of lipid peroxides could have contributed to and increase in the enzymeactivities, indicating their protective role in maintaining membrane integrity. Ca++ ATPase activity was found to be decreased in cancer bearing animals. Free intracellular calcium, acting as a second messenger, is crucial for a diverse range ofbiological functions (Berridge et al., 2000). Intracellular calcium signaling is also a key regulator of proliferation Lipskaia (2004), cell cycle progression and apoptosis Orrenius (2003). The plasma membrane Ca++ ATPase (PMCA) or pump belongs to the family of P-Type ATPases and is a critical regulator of free intracellular Ca++ . There are two isoforms of PMCA (PMCA1-4) Carafoli (1994). PMCA alterations are also found to be associated with tumorigenesis. The decrease in the activity of cancer bearing animals suggest that there is a high concentration of Ca++ inside the cells due to toxicity created by DEN, which the calcium pump tries to eliminate, to keep its level low. Subsequent increase in the activity was recorded after treatment with UA suggesting its protective role. Further Ca++ ATPase activity is mainly impaired due to oxidative modification of thiol groups present in this enzyme which in turn is due to the generation of free radicals (Jain and Sohet, 1981). The fact that UA has been found to reduce protein carbonyls in our study thereby reducing stress and free radical formation might be a reason in restoring the enzyme activity. It can be deciphered that ursolic acid conditions hepatic cells, preventing any further damage to liver parenchyma which in turn would have decreased the leakage of enzymes into circulation. Further, since ursolic acid has been reported to have high binding capacity to liver, it can be assumed that quicker regeneration of hepatocytes and hepatic parenchyma would have occurred on administration of membrane bound enzymes and decrease the levels of lipid peroxides, glycoproteins and protein carbonyls thus restoring the membrane integrity and suggesting its role as a chemopreventive anticancer and antioxidant compound. Acknowledgement One of the authors, Gayathri R acknowledges the University of Madras, Chennai, India, for granting financial assistance in the form of University Research Fellowship. The author also wishes to acknowledge the effort and support provided by Mr. R. Baskar Raman in manuscript compilation. References Ana I, Galan M, Munoz E, Jimenez R (1999). S- Adenosylmethionine protects against cyclosporin A- induced alterations in rat liver plasma membrane fluidity and functions. J Pharmacol Exp Ther, 290, 774-81. Berlett BS, Stadtman ER (1997). Protein oxidation in ageing, disease and oxidative stress. J Biol Chem, 272, 20313-6. Berridge MJ, Lipp P, Bootman MD (2000). The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol, 1, 11-21. Bhosale P, Motiwale L , Ignle AD, Gadre RV, Rao KVK ( 2002). Protective effect of Rhodotorula glutini – NCIM3353 onthe development of hepatic preneoplastic lesions. Curr Sci, 83, 303-8. Bonting SL (1970). In: Bittar EE, ed. Membranes and ion transport. London: Wiley Interscience, pp. 257-263. Carafoli E (1994). Biogenesis: Plasma membrane calcium ATPase: 15 years of work on the purified enzyme. Faseb J, 8, 993-1002. Chaterjee SN, Agarwal SN (1988). Liposomes as membrane model for study of lipid peroxidation. Free Radic Biol Med, 4, 51-72. Contreras RG, Shoshani L, Flores-Maldonado CA, Lozaro A, Cereijido M (1999). Relationship between Na+K+ ATpase and cell attachment. J Cell Sci, 112, 4223-4232. Crook M (1993). The determination of plasma or serum sialic acid. Clin Biochem, 26, 31-8. Cunningham ML (1996). Role of increased DNA replication in the carcinogenic risk of nonmutagenic chemical carcinogens. Mutat Res, 365, 59-65. Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milazani A (2006). Biomarkers of oxidative stress in human disease. Clin Chem, 52, 601-23. Dennis JW, Granovsky M, Warren CE (1999). Glycoprotein glycosylation and cancer progression. Biochem Biophys Acta, 473, 21-34. Dich, J, Zahm SH, Hanberg A, Adami HO (1997). Pesticides and cancer. Cancer Causes Control, 8, 420-43.and cancer. Cancer Causes Control, 8, 420-43. Dodge JD, Mitchell G, Honatian DJ (1963). The preparation and chemical characteristics of hemoglobin free ghosts of human red blood cells. Arch Biochem Biophys, 180, 119-30. Dragan YP, Hully JR, Nakamura J, et al (1994). Biochemical events during initiation of rat hepatocarcinogenesis. Carcinogenesis, 15, 1451-8. El-Serrag H (2004). Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol, 35, 572-8. Erbil K, Jones JD, Klee GG (1985). Use and limitations of serum total and Lipid-bound sialic acid concentrations as markers for colorectal cancer. Cancer, 55, 404-9. Fiske CH, Subbarow Y (1925). The colorimetric determination of phosphorous. J Biol Chem, 66, 375-400. Goldsworthy T, Hanigan M, Pitot H (1985). Models of hepatocarcinogenesis in the rat - contrasts and comparisions. CRC Crit Rev Toxicol, 7, 61-89. Hjerten S, Pan H (1983). Purification and characterisation of two forms of a low affinity calcium ion ATPase from erythrocyte membrane. Biochem Biophys Acta, 28, 281-8. Huber WW, Grasl-Kraupp B, NAME?, et al (1997). TITLE? Arch Toxicol, 71, 575-81. Jain KS, Sohet SB (1981). Calcium potentiates the peroxidation of erythrocyte membrane. Lipids, 642, 46-54. Jewell SA, Bellomo G, Thor H, Orrenius S, Smith MT (1982). Bleb formation in thiol and calcium ion homeostasis. Science, 217, 1257-59. Kang CB, Lee ES, Hur JH (1997). Effects of administration of CCl4 on liver function in rats. Kor J Vet Clin Med, 14, 273- 278. Kim JS, Huh JI, Song SH, et al (1996).The antioxidative mechanism of ursolic acid. Kor J Gerontol, 6, 52-6. Kokoglu E, Sonmez H, Uslu E, Uslu I (1992). Sialic acid levels in various types of cancer. Cancer Biochem Biophys, 13,57-64. Kunimoto M, Inoue K, Nojima S (1981). Effect of ferrous ion and ascorbate induced lipid peroxidation on liposomal membranes. Biophys Acta-Biomembranes, 646, 169-78. Kurtul N, Cil MY, Bakan E (2004). The effects of alcohol and smoking on serum, saliva, and urine sialic acid levels. Saudi Med J, 25, 1839-44. Lee IN, Chen CH, Sheu JC, et al (2005). Identification of Human Hepatocellular carcinoma related Biomarkers by two dimensional difference gel electrophoresis and mass spectrometry. J Proteome Res, 4, 2062-9. Levine RL, Garland D, Oliver CN, et al (1990). Determination of carbonyl content in oxidatively modifiedproteins. Methods Enzymol, 186, 464-478. Li B, Cao CP, Mao GP (2005). Effect of proapoptosis protein on hepatocarcinogenis. Chin J Dig Dis, 6, 93-7. Lindberg G, Rastam L, Gullberg B, Eklund GA, Tonberg S (1991). Serum sialic acid concentration and smoking: A population based study. BMJ, 303, 1306-7. Lipskaia L, Lompre AM (2004). Alteration in temporal kinetics of Ca++ signalling and control of growth and proliferation. Biology Cell, 96, 55-68. Lodato F, Mazzella G, Festi D, et al (2006). Hepatocellular carcinoma Prevention: a worldwide emergence between the opulence of developed countries and the economic constraints of developed nations. World J Gastroenterol, 12, 7239-49. Loppen S, Schneider D, Gaunitz F, et al (2002). Title??? Cancer Res, 62, 5685-8. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951). Protein measurement with the Folin-phenol reagent. J Biol Chem, 193, 265-75. Mandal TK, Chaterjee SN (1980). Ultraviolet and sunlight induced lipid peroxidation in liposomal membranes. Radiat Res, 83, 290-302. Martin-Aragon S, De las heras B, Sanchez-Reus MI , Benedi J (2001). Pharmacological modification of endogenous antioxidant enzymes by ursolic acid on tetrachloride induced liver damage in rats & primary cultures of rat hepatocytes. Exp Toxic Pathol, 53, 199-206. Michejda CJ, Kroeger-Koepke MB, Koepke SR (1982). Nitrogen formation during in vivo and in vitro metabolism of N-Nitrosamines. In: Magee PN, ed. Nitrosamines and human cancer Banbury report, Cold Spring: Cold Spring harbor laboratory, 12, 69-81. Moore M, Kitagawa T (1986). Hepatocarcinogenesis in the rat: the effect of promoters and carcinogens in vivo and in vitro. Int Rev Cytol, 101, 125-173. Nakae D, Kobayashi Y, Akai H, Nabuaki A (1997). Involvement of 8- hydroxyguanine formation in the inhibition of rat liver carcinogenesis by low dose levels of N-nitrosodiethylamine. Cancer Res, 57, 1281-1287. Neibes D (1972). Determination of enzymic and degradation products of glycosaminoglycan metabolism in the serum of healthy and various subjects. Clin Chem Acta, 42, 399-408. Ohinishi T, Suzuki T, Suzuki Y, Ozawa K (1982). A comparative study of plasma membrane magnesium ion ATPase activities in normal, regenerating and malignant cells. Biochem Biophys Acta, 684, 64-74. Ohkawa H, Ohishi N, Yagi K (1979). Assay for lipidperoxides in animal tissue by thiobarbituric acid reaction. Anal Biochem, 95, 351-358. Parkin DM, Pisani P, Ferlay J (1999). Global cancer statistics. CA Cancer J Clin, 49, 33-64. Pitot H (1986). Fundamentals of Oncology, 3rd edition. New york: Marcel Dekker. Ponio M, Alho A, Heinala P, Nikkari ST, Sillanaukee P (1999). Serum and saliva levels of sialic acid are elevated in alcoholics. Alcohol Clin Exp Res, 23, 1060-64. Qi Y, Chen X, Chan C-Y, et al (2008). Two-dimensional differential gel electrophoresis/analysis of diethyl- nitrosamine induced rat hepatocellular carcinoma. Int J Cancer, 122, 2682-8. Quist EE (1980). Regulation of erythrocyte membrane shape by calcium ion. Biochem Biophys Res Commun, 92, 631-7. Saravanan R, Viswanathan P, Pugalendi KV ( 2006). Protective effect of ursolic acid on ethanol-mediated experimental liver damage in rats. Life Sci, 78, 713-8. Singer B, Greunberger D (1983). Alkylation of nucleic acids in vivo. In: Singer B and Grunberger D, eds. Molecular Biology of Mutagens and Carcinogens, New York: Plenum Press, 68-78. Stadtman ER (1992). Protein oxidation and ageing. Science, 257, 1220-4. Subbaramaiah K, Michaluart P, Sporn MB, Dannenberg AJ (2000). Ursolic acid inhibits cyclooxygenase-2 transcription in human mammary epithelial cells. Cancer Res, 60, 2399-404. Swenberg JA, Hoel DG, Magee PN (1991). Mechanistic and stastical insight into the large carcinogenesis bioassays on N-nitrosodiethylamine and N-nitrosodimethylamine. Cancer Res, 51, 6409-14. Tian Y, Lin G, Zheng R-X, et al (2006). Anti hepatoma activity and mechanism of ursolic acid and its derivatives isolated from Araliadecaisneana. World J Gastroenterol, 12, 874-9. Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J (2004). Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem, 266, 37-56. Verna L, Whysner J, Williams GM (1996). N- nitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther, 71, 57-81. Wagner WD (1979). More sensitive assay discriminating galactosamine and glucosamine in mixtures. Anal Biochem, 94, 394-7. Warren L (1959). The thiobarbituric acid assay of sialic acid. JBiol Chem, 234, 1971-1975. |







 Ursolic Acid Attenuates Oxidative Stress-mediated Hepatocellular Carcinoma Induction by Diethylnitrosamine in Male Wistar Rats
Ursolic Acid Attenuates Oxidative Stress-mediated Hepatocellular Carcinoma Induction by Diethylnitrosamine in Male Wistar Rats 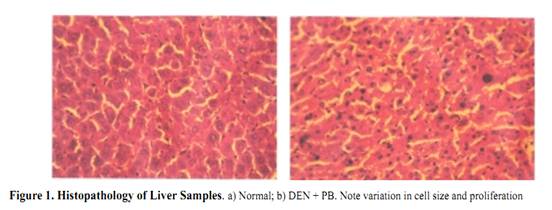
 Results
Results