| Icariin from Epimedium brevicornum attenuates chronic mild stress-induced behavioral and neuroendocrinological alterations in male Wistar rats |
| 发布时间:2012-08-02 信息来源:admin 发布人:admin 点击次数:10243 |
|
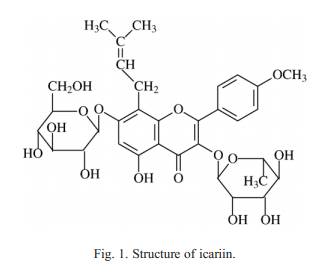
Abstract Chronic mild stress (CMS) is suggested to produce abnormalities in the hypothalamic – pituitary– adrenal (HPA) axis and hypothalamus –pituitary– thyroid (HPT) axis. Therefore, compound that attenuates the neuroendocrinological alterations may have potential as antidepressant. The behavioral and neuroendocrinological effects of icariin, a major constituent of flavonoids isolated from Epimedium brevicornum, were investigated in the CMS model of depression in male Wistar rats. CMS procedure caused an anhedonic state in rats resulted in increased corticotropin-releasing factor (CRF) concentrations in dissected brain regions and serum, decreased total triiodothyronine (tT 3 ) in serum with no significant changes in serum adrenocorticotrophic hormone (ACTH) and thyroxine (tT 4 ). Administration of icariin reversed CMS-induced sucrose intake reduction and CRF elevation. These results suggested that icariin possessed potent antidepressant-like activities which were at least in pa rt mediated by improving the abnormalities in the HPA axis functions. However, we did not find a clear correlation between the HPT axis and icariin treatment in the CMS-treated rats. Keywords:Icariin; Chronic mild stress; Hypothalamic–pituitary–adrenal axis; Hypothalamus–pituitary–thyroid axis 1. Introduction Depressi on invol ves pathop hysiologi cal changes in ne uro-endocri nological funct ion (Muss elman and Nemeroff, 19 96;Helmr eich et al., 2005; Tichom irowa et al., 2005). The most frequently occurring neuroendocrinological abnormality in depressed s ubject s i s hyperactivity of the hypothalamic –pituita ry–adrenal (HPA) axis characteri zed by hypers ecret ion of cort icotropin-releasing f actor (CRF), w hich s tim ul at es adrenoc orticotr ophic horm one (ACTH) release ( DeMoranvi lle and Jackso n, 1 996; Tsigos and Chrousos , 2002; Barden, 2004 ). Althoug h, in c ontrast to the HPA axis syst em, overt hypoth al-amus–pitu itary–thyro id (HPT) dysfun ction is not comm on in depres sion ( Fountou lakis et al., 2004; Sch ule et al., 2005a,b ), thyro id hormones have a profou nd influe nce on behavi or and appear to be ca pable of modulati ng the phenot ypic expres sion of major affective illness (Muss elman and Neme roff, 1996; Bauer and Whybro w, 2001; Fountou lakis et al., 2004 ). Signi ficant reduction in serum total triiodothyron ine (tT3) concent rations but not in total thyro xine (tT4) concent rations was observ ed in depressed pati ents ( Rub in et al., 1987; Sakaue, 1990 ). How ever,increased serum tT3and tT4levels wer e found in chronic mild stress-induced Sprague– Dawle y and Wist ar rats with no any change in p lasma ACTH levels ( Kioukia -Fougi a et al., 2000 ). In addition, there was a close inte rrelatio nship between the HPA and the HPT axes in d epression (Baumgar tner et al., 1990; Helmreich et al., 2005). CRF suppre ssed thyro id functions (Tsigos and Chr ousos, 2002 ), resul ting in T 3 content reduction. Converse ly, CRF release in the h ypothalam us increased in hypothy roid animals ( To hei et al., 199 8 ). Eliminat ion of thyroid hormones caused a mark ed reduct ion in transcrip tion of CRF gene in the paraven tricular nucleu s of mal e rats , sugges ting that the horm ones of the HPT axis had a major effec t on the centra regul ation of the HPA axis ( Shi et al., 1994). It is importan t to note that the compl ex regul ation of the HPA and HPT axes highlight s organisms compl icated adjus tability to stre ssful sit -uation s ( Helmr eich et al., 2005). The neu roendoc rinologi cal abnorm alities of the HPA and HPT axe s, alth ough not o bserved in all patients with depres sion ( Fava et al., 1995 ; Watson et al., 2002; Fountou lakis et al., 2004), have been identifi ed as a useful diagno stic tool. Clinical studies also provi ded eviden ce that norm alizatio n of the HPA ax is or the HPT a xis abnorm alities preceded succes sful treat ment with anti depressants ( Joffe, 1992; Bschor et al., 2003; Young et al., 2004; Nikisc h et al., 2005; Rao et al., 2005; Sc hule et al., 2005a,b ), indicati ng that future anti-depres sants might target the ne uroendo crinologi cal systems by regul ating either the HPA axis or the HPT axis. Icariin (Fig. 1) is a maj or constitu ent of flavon oids isol ated from Epim edium brevicor num Maxi m (B erberidaceae ), which is used in tradi tional Chinese medicine to no urish the kidney and reinforce yang . Clini cal eviden ces suggested that E. brevicor-num and its decoct ion could imp rove depres sive symptom s after stroke ( Lai, 200 1; Ma, 2003). E. b revicor numdecreas ed plasma ACTH and corticost erone c oncentrati ons and posses sed prote c-tive effects on hypothalam us–pitu itary– adrenal – thymus axis induce d by ex ogenous glucoc orticoid in clin ical and experi -ments (Cai et al., 1998). The total flavo noids reversed the at-tenuations of m o noam ine n eurotransmitters and regulated neuroen docri ne–immuno logical network in hypo thalamus of the old rats ( Shen et al., 2004 ). Recently, our labor atory demon -strated a ntidepress ant actions of total flavo noid extracts and icariin from E. brevicor numin the forced swimming test (FST ) and the tail suspen sion test (T ST) in mice (Pan et al., 2005; Zhong et a l., 2005 ). Subseque nt study exh ibited that icari in admin istration attenua ted the swim stress-induce d e levation in serum CRF concent rations (Pan et al., 2005). Thu s, these findings might provi de some suppor ts for the hypoth esis that icariin could modul ate abnorm al neuroen docri nological func-tion in depres sed anim als. Chr o nic m ild str es s (CM S) m od el o f de p r e ss i on in a ni m al i s accepted as a v al ua bl e m ethod for predicting potential an ti-d ep r e s s an t a ct i on s o f c om po u nd s in hu ma n s . Th e CM S r e gi -me n a lte re d b eh av io ra l pa r ame t e r s c o ns i st en t w ith a l os s o f responsiveness to reward, such as d ec reas ed sucrose consump-ti on, a specific he donic d ef icit ( Will ner et al ., 1 987; Wi llner, 1997, 2005 ). The s tress-i nduced anhedonic-like st ate in rat s
gr ad u all y d ev el op e d ov e r se ve ra l w e ek s a nd c o uld be pr e-ve n t ed or re ve rs ed by ch ro ni c a dm ini s tr at io n o f an tid e pr es s a nt dr ug s ( G riebel et a l., 2002; P app e t al., 2 003; Willner, 2005 ). In addition, t he C M S produced several n eurohormon al changes in rode nts t ha t a re similar to those foun d in human depr es sion ( Azpi roz e t a l., 1 999; Brat t et al., 2001; Kiouk ia-Fougia et al., 20 02; Grippo et a l., 2 005a). However, few s tudies have si-mu lta ne o us l y e xa mi ne d b eh a vio ra l a nd ne ur oe n do c rin ol og i c al ch anges i n the HPA a nd the HPT axes during the CMS ex po-sure in rats. The present s tudy explores the p os sible r el a-tions hip o f the HPA a xi s and the HPT axis in the CMS model of depressi on in male Wist ar rats , a nd s i mu lt an eo u sly ex am in es the e ff ects of icarii n a nd know n antidepressant fluoxetine o n ne urohormonal mediators of t he H PA axis (circulating CRF an d ACTH), as well a s t he central n ervous system (CRF in the di ssected brain) an d t he HPT a xis ( ci rculating tT3and tT4). These results first ly d em onst rate t hat the C M S i nduces a pr of ile of the two ax es alterations i n rats and provide a basis f or ex a m in in g t h e n e ur oh or mo na l p at hwa ys di r e ct l y a nd in te ra c-tions that underlie the li nk b etw ee n d e pr es sio n an d i ca ri in treatment . 2. Materi als and methods 2.1. Anim als Male Wistar rats (Laboratory Animal Center, Jiangsu Prov-ince, China), weighing 220–250 g, were brought into the lab-oratory 3 weeks before the experiment started. The animals were individually housed, with food and water freely available, and maintained on a 12 h dark–light cycle (with the lights on at 07:00 h locate time) under regulated temperature conditions (22 ±2 °C), except as described below. The study was approved by the institutional Animal Care Committee at the Nanjing University, or the China council on Animal Care at Nanjing University. 2.2. Dru gs Icariin was purchased from Bio-se p Bio-t echnique Stock Co., Ltd. Xian Jiaotong Univers ity (P. R. China). The purity of icariin was checked by high-perfor mance liqu id chrom atogra-phy to be at least 98% pure ( Wang et al., 2003). Fluoxetine hydroch loride was from Cha ngzhou Siyao Pharm aceutic als Co., Ltd. (P. R. China). Other reagent s were analytical grades made in P. R. Chin a. 2.3. Chronic mild stress (C MS) Before CMS procedu re, rats wer e trained to consum e a 1% sucrose solut ion. Traini ng consisted of initial 72 h sucros e solution expo sure without any food or water available. After the period of adaptation, anim als w ere d istributed into two subgro ups and sucros e solution intake basel ine tests were performed 6 time s over 14 days for all subje cts. Sucrose intake tests took place once a week at regular times. The intake was expres sed in relation to the anim als body wei ght (g/kg ).These tests invol ved a 14-h period of food and water depri vation Fig. 1. Structure of icariin. 131 Y. Pan et al. / Pharmacology, Biochemistry and Behavior 87 (2007) 130–140 follow ed by the offering of a sucros e solution for 1 h. At the end of each test, sucros e intake was meas ured by weighting pre-weighed bottles containing the sucros e solution . Subseque ntly, sucros e consum ption was moni tored, under simil ar condit ion, in 1 h tests (11:00 – 12:00 h), at week ly inte rvals for the next 10 week s. On the basis of their sucros e inta kes in the final basel ine test, the anim als were random ly divi ded into two groups ( n =40 in every group) having simil ar average inta ke. One group was subjected the chronic m ild stress ( CMS-treated animals) procedu re. The CM S procedu re was slightl y modified from that previ ously described by Willner et al. (1987) and Papp et al. (2002) . The week ly stress regime consi sted of one perio d (12 h) of paire d caging , two perio ds (14 a nd 18 h ) of tilted cage (45°), two perio ds of water and food d eprivatio n (14 and 18 h), one 12-h perio d wi th wet cage (200 ml wat er in 100 g saw dust beddin g), and two perio ds (12 and 12 h) of continuous ligh t, three periods (6, 10 and 12 h) of low inte nsity stro boscopic illuminati on (150 flashes/min ), one 12-h perio d of inte rmitt ent illuminati on (2-h/2-h ligh t/dark cycle), tw o pe riods of noise (6300 Hz tone, 10 a nd 12 h). All of the stre ssors wer e applied individua lly and continuous ly, day and night . The other group was housed in separate room s and had n o contac t wi th the stressed animals. The se rats were depri ved of food and water for the 14 h preceding each sucros e test, but otherwi se food and water were freely avail able in the home cage. On the basis of sucros e inta ke scores foll owing 5 week s of the stress, both stressed and unstressed animals were further divided into mat ched subgro ups. Di fferent groups of anim als ( n = 8 in e very group) were adminis tered with vehicl e (sal ine 1 ml/kg per day), icariin (15, 30 and 60 mg/kg per day), and fluox etine (10 mg/ kg per day), respec tive ly. All drugs were suspen ded in a 0 .9% norm al saline, and wer e adminis tered by gavage o nce daily at 13:00 h follow ing the weekly sucrose intake test (appro xim ately 1 h later) for the subseq uent 5 weeks. Stres s was conti nued throughout the enti re treat ment period. 2.4. Collection of blood After the CMS perio d and post- CMS sucros e intake test, rats were left without any treatmen t until the foll owing morn ing. To avoid fluctuati ons on hormone concent rations due to circad ian rhyth ms, animals wer e sacri ficed via decapi tation between the hours o f 09:00 and 10:00 h on two consecu tive days. Blood was collected in pre-i ced tubes wi th prote ase inhi bitor aprotinin and centr ifuged at 3000 rpm at 4 °C for 20 min . The separa ted serum samp les wer e stor ed at − 80 °C until the assay of CRF, ACT H,tT3and tT4, respec tively. 2.5. Collection of brain tissues Immedia tely follow ing decapi tation, the cortex, hippoc am-pus, corpus striatum and medul la oblong ata in anima l brain were disse cted out, an d placed into pre-weig ht plast ic chil led tubes treated with aprot inin. The wet wei ght of the organs was then determin ed by subtr action of the weigh t boat alone, and repres ented as wet wei ght. Tissues (about 30 mg) were boiled
for 3 min in 1 ml saline solution , and then homog enized in 0.5 ml of 1 M acetic acid at 4 °C using an ultrasoni c cell disrupter, set at 4 °C for 1 h. 0.5 ml of 1 M NaOH was add ed and centrifuge d at 15,000 rpm for 20 min at 4 °C ( Vale et al., 1983; Tang et al., 1995 ). Clear supern atants were collected. Recovery ranged between 80 and 90% for ex tracted CRF. The supern atant was stor ed at − 80 °C unti l assaye d for CRF. The meas ured immunor eactive values for CRF in brain regio ns wer e expres sed in ng/g wet tissue wei ght (ng/g ww ). 2.6. Neu rohorm one assay CRF, serum ACTH , serum tT 3and serum tT4 concent rations were determin ed in dupli cate by radio immunoa ssay (R IA), using commerci ally available RIA kits which were manuf ac-tured by Techniq ue Center of Radioi mmunit y of Navy in Beijing, P. R. China. The sensi tivities of these assay kits were 0.2 ng/m l for CRF, 5 pg/ml for ACT H, 0.2 5 ng/ml for tT3 and 3 ng /ml for tT4, the intr a- and inte r-assay coefficients of variation were less than 8% and 12% for CRF, less than 6% and 12% for ACTH , less than 10% and 15% for tT 3, less than 10% and 15 % for tT 4 . The RIA procedu re was perfor med as described by the kits manuf acturer, respec tive ly. 2.7. Dat a analys is Values wer e presen ted as mean ± stand ard error of the mean (SEM) for the indicated analys es. Sucrose inta ke data were analyzed by multip le analys es of varia nce (ANO VA) wi th drug treatmen ts in stress and unstressed groups as between-s ubjects factors and sucros e test week as wi thin-subje ct factor. Data for biochemic al param eters also used ANOVA with drug treat ment in stressed and unstr essed groups as between-s ubjects factors. In all analysis the LSD test or Dunn etts T3 test was used for post-
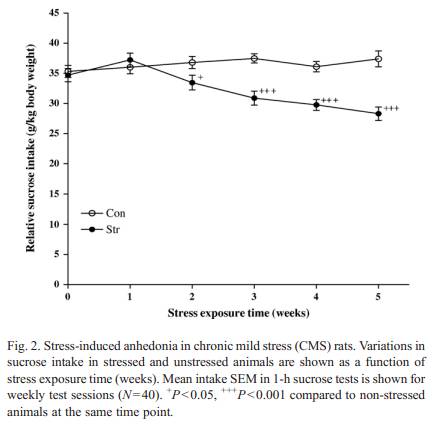
hoc c omparison s of means . P -values lower than 0.05 were consi dered to be statistically signifi cant. 3. Results 3.1. Sucrose intake The effects of the CMS regimen on inta ke were shown in Fig. 2. Before stre ss, intake values for control and stre ssed groups were 3 5.33 and 34.70 g/kg body weig ht, respec tive ly. Compar isons of data obtained in stre ssed and non-st ressed anima ls revealed a signi ficant stre ss effect [we ek 2: F (1, 78) =4.446, P = 0.038; week 3: F (1, 78 ) = 22.280, P = 0.000; week 4: F (1, 78 ) = 26.321, P = 0 .000; week 5: F (1,78) = 27.918,P = 0.000] from week 2 to week 5 of the experi ment. In stre ssed anima ls, inta ke progre ssive ly decreased over a perio d of about 2 week s o f CM S and then remained consi stently low throu ghout the rest stress period. At week 5 of stre ss regimen, intake values for contr ol and stressed anim als were 37.40 and 28.30 g/kg body weight, respec tive ly, resulting in a signifi cant Group (cont rol/ stressed) effect [ F (1, 78) = 33.256, P b 0.001]. Such a difference between contr ol and stressed anim als treated with vehicl e, pe r-sisted for the rema inder of the 5-w eek treat ment perio d.
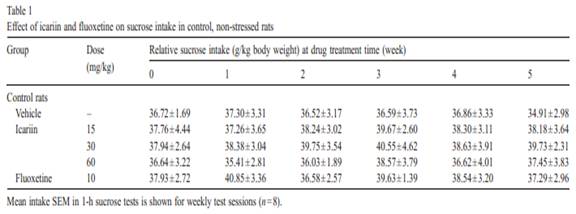
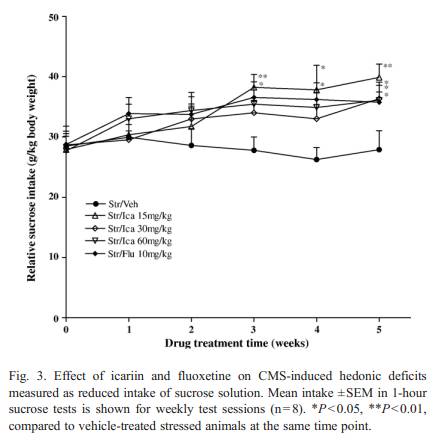
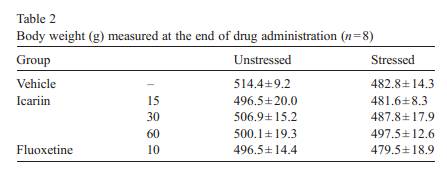
In unstressed anim als, the sucrose inta ke was not changed by treatmen ts of three doses of icariin [ F (3, 168) = 0.904,P = 0.853] or by treatmen t of fluox etine [F (1, 84) = 1.375,P = 0.244] ( Table 1 ).In stressed animals, icar iin caused a gradual increase ofsu cr os e i nt ak e, r es ult in g i n a sig n i fi ca n t effe c t o f ic ar iin tr ea t-ment [ F (3, 28) = 3.438, P =0.030] and Week [F (4, 112) =2.548, P =0.044] ( Fig. 3) . Co mp ar ed to wee k 0 s co re s ( th e l a stscor es before drug treatment), increas e in sucrose intake wasapparent during the first 2 week s o f t reat ment, a nd reac he d statistica l s ignificance a t wee k3instressedanimalsreceiving 15 mg/kg o f icariin [F (1, 14) = 11.07 0, P = 0 . 0 0 5 ] . Th i s i n -cr ea se effect was maintained, and a t week 5 the sucrose intake by t he se an i ma ls wa s c om pa ra bl e t o t ha t of v eh ic le -t re ate dcontrols, and significantly hi gh er than that of vehicle-treated stressed animals [F (1, 14) = 9.848 , P = 0 .007]. For the 3 0 and 60 mg/kg icariin-treat ed stressed groups, some significa nt effec t s a pp e ar ed af t e r 5 w ee ks of dr ug tre a t me nt [3 0 m g / k g:F (1, 14) = 5.1 07, P = 0.041; 60 mg/kg: F (1 , 14) = 5.02 8,P = 0 .0 43 ]. Compared to week 0 scores, the sucrose intake in fluoxetine-treated str es se d a n i ma ls was si gn i f i ca n tly in cr ea se daf ter 3 weeks of treatment [ F (1, 1 4) = 5.208, P = 0.039] and af te r 5 w ee ks of t re a t me nt th er e w e r e no s i gn i f i ca n t di ff er en c es betw een drug-treated st ressed a nd v e hi cl e- tr ea te d co n t r ol an ima l s [ F (1, 14) = 0.202, P = 0.660]. 3.2. Bod y wei ght Table 2showed that at the end of the treatmen t perio d the vehicle-t reated stre ssed anim als wer e smaller in body weight than the vehicle-t reate d contr ol animals but this difference was not signifi cant [ F(1, 14) = 3.428, P = 0.085]. As compa red to vehicle-t reated groups, body weights of contr ol and stressed animals wer e not signifi cantly affected by icariin [Contr ol: F(3,28) = 0.229, P = 0.876; Stressed: F(3, 28) = 0.229, P = 0.841]
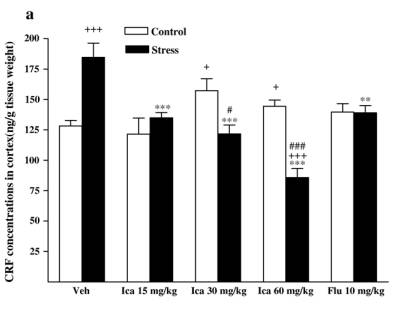
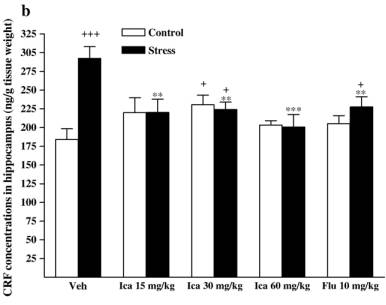
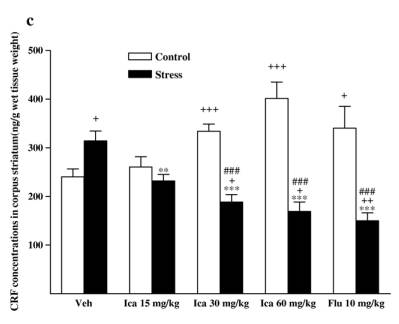
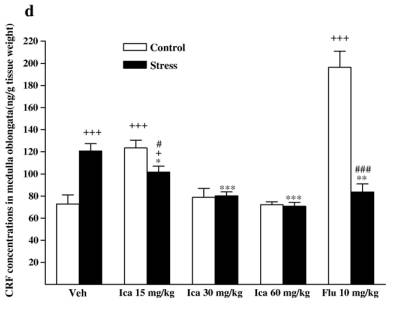
while fluox etine caused a slight but insignif icant de crease in stressed animals [F (1, 14 ) = 1.091,P = 0.314]. 3.3. Neurohorm onal concent rations 3.3.1. CRF Followi ng exposure to the CMS procedu re, CRF concent ra-tions wer e markedly incre ased compa red with vehicle-t reated contr ol rats in diss ected brain regio ns and serum [cort ex: F(1,14) = 20. 158, P b 0. 001; hippo campus : F (1, 14) = 20.1 58,P b 0 .001; corpus striatum: F(1, 14) = 8.032, P = 0. 013; medul la oblong ata: F(1, 14) = 21.041, P b 0.001; hypothalam us: F(1,14) = 29.820, P b 0.001; serum: F(1, 14) = 11.648, P = 0.004].The effects wer e reduced by treatmen t wi th icariin or fluox etine in anim als subje cted to the CMS procedu re (Fig. 4). 3.3.1.1. Cortex. Icariin-treated stressed subjects showed a sig-nificant decrease in CRF [ F( 3 , 2 8) = 2 5. 34 1, P b 0.001]. Icariin treatment at 60 mg/kg decreased CRF concentrations below that of th e un stre ssed con trol . Ho weve r, icar iin- tre ated con troll subjects showed a significant increase in CRF [ F( 3 , 2 8) = 3 .3 03 , P = 0.035]. Icariin at 30 and 60 mg/kg significantly increased CRF compared to the vehicle control group. Fluoxetine treatment showed a significant decrease in CRF in stressed animals [ F(1,14) = 12.019,P =0.004 vs.vehicle-treated stressed animals], but no change in control animals [ F( 1 , 1 4) = 1 .9 42 , P =0.185 vs. vehicle-treated control animals] ( Fi g. 4a). 3.3.1.2. Hippocam pus. Icariin-treated stressed subjects showed a significant decrease in CRF compared with vehicle-treated stressed anim als [F (3 , 28) = 6 .94 2 , P b 0.001]. C ontrol anima ls only r eceivin g 30 mg/kg dose of i cariin significantly elevated CR F [F (1, 14) = 5 .870, P = 0.030]. Fl uoxetine elicit ed to de-crease CR F in stressed an im als [ F (1, 14) = 9.817 , P = 0.007] but fail ed to affe ct the control r ats [ F (1, 14) = 1.429, P = 0 .252] ( Fi g . 4b). 3. 3.1 .3 . Cor pu s s tri a tum . I ca rii n -tre ate d st res sed sub je cts showed a signifi cant dec rease in CRF [ F (3, 28) = 13.865, P b 0.001]. A slight positive dose-d ependent decreas e in CRF was observ ed in the icari in-treated stre ssed anim als. Icariin treatmen t at 60 mg/kg reduced CRF below that of the control.
Fig. 4. Effects of icariin (Ica, 15, 30 and 60 mg/kg) and fluoxetine (Flu, 10 mg/kg) on CRF concentrations of dissected brain regions and serum in unstressed and CMS-treated rats. a) In cortex, b) in hippocampus, c) in corpus striatum, d) in medulla oblongata, e) in hypothalamus, f) in serum. Values are means ± SEM.*P < 0.05,**P < 0.01,***P < 0.001, compared to vehicle-treated stressed animals;+P < 0.05,++P < 0.01,+++P< 0.001, compared to vehicle-treated control animals.#P < 0.05,##P < 0.01,###P <0.001, compared to appropriate drug-treated control animals. However, icariin- treated contr ol subjects showed a signifi cantincre ase in CRF [ F (3, 28) = 10.607, P b 0.001]. The positivedose-d ependent incre ase was also observ ed in the icariin- treated contr ol anima ls. Fluoxetine- treated stressed rats reveal ed a decrease in the c oncentrations below t he vehicle-treated stressed rats [F(1, 14) = 40.036, P b 0.001]. Fluoxet ine treatmen t induce d CRF rise in control rats [F (1, 14) = 4.393, P = 0.055 ]( Fig. 4c). 3.3.1.4. Medul la oblong ata. Icariin-treat ed stressed subje cts showe d a signi ficant decreas e in CRF [ F(3, 28) = 20.516,P b 0.001]. Stres sed animals receiving 30 and 60 mg/ kg o f icariin could revers e CMS-in duced CRF increase to the norm al.However, icariin- treated unstr essed subje cts showed a signifi -cant incre ase in CRF [ F(3, 28) = 13.011, P b 0.001]. The un stressed animals only receiving a dose of 15 mg/ kg icari in signifi cantly incre ased the CRF compa red with the vehicle contr ol g roup. Fluoxetine treat ment signifi cantly decreased the concent rations in stressed rats [ F(1, 14) = 14.201, P = 0.002]. In contrast, it rema rkabl y elevated CRF in contr ol animals [ F(1,14) = 54.348,P b 0.001 ] (Fig. 4d). 3.3 .1.5 . Hy potha lamus. Icariin-treated s t ressed subjects showed a signifi cant decreas e in CRF compa red wi th vehicletreated stre ssed animals [ F(3, 28 ) = 20.665, P b 0.001]. Icariin treatmen t at the lowest d ose decreas ed CRF below the unstressed control. The re was no statistical signifi cance of icariin treatment on CRF in unstressed animal. Fluoxet ine significa ntly reduced the concent rations in stre ssed anim als [ F(1, 14) =23.256,P b 0.0 01] but did not alte r that in control anima ls [ F(1,14) = 0.112 , P = 0.742] ( Fig. 4e). 3.3.1.6. Serum. Icariin-treat ed stressed subje cts show ed a significant decreas e in CRF [ F(3, 28) = 6.047, P = 0.003]. In unstressed animals icariin merely at 15 m g/kg significantl yincreased CRF compa red to vehicle-t reate d contr ol [F (1, 14) =5.675, P = 0.032] (Fig. 4f). However, fluox etine failed to be
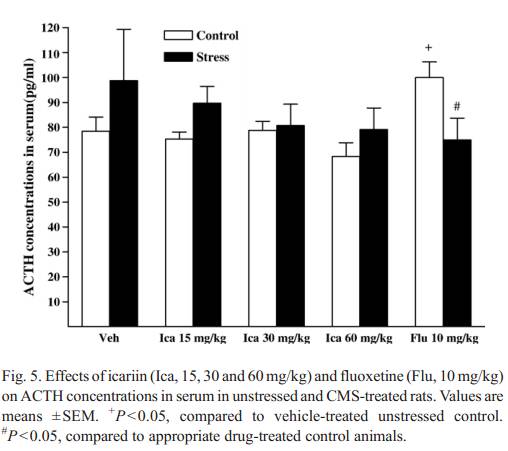
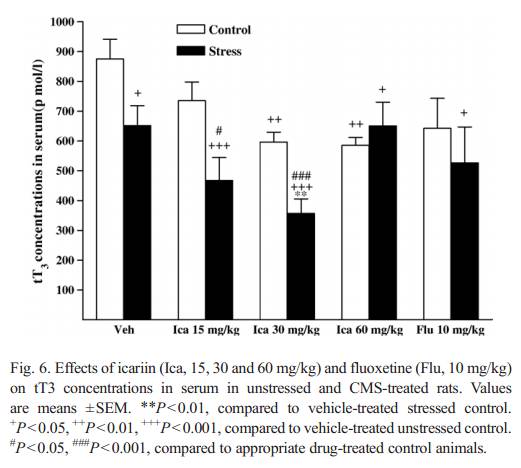
effect both in stre ssed and unstressed animals compared to the corres ponding vehicle groups . 3.3.2. ACTH. No stress effect was observed in serum ACTH concentrations (F( 1 , 1 4) = 0 .8 99 , P = 0.359). There was no signifi-cant interaction between icariin treatment and group [F(3, 56) =0.334, P = 0.801). However, fluoxetine produced a significant increase in ACTH in unstressed groups but no change in stressed animals compared to the corresponding vehicle groups (Fi g. 5). 3.3.3. tT3 As show n inFig. 6, rats subje cted to the CMS procedu re,showed a s ta tistically significant d ecrease i n s erum tT3compa red to the unstressed veh icle groups [ F (1, 14) = 5.573,P = 0 .033]. The signifi cant inte raction betw een icariin treatment and group was observ ed [ F (3, 56) =3.302 , P = 0.027]. Icari in-treated stre ssed subjects reveal ed a signifi cant reduct ion in serum tT3 concent rations [ F(3, 28) = 4 .401, P = 0.012]. Icari intreatmen t at 30 mg/kg decreas ed tT3 below the vehicle-t reated stressed contr ol [F(1, 14) = 12.954, P = 0.003]. Additi onally,serum tT3 in stressed an imals recei ving icariin a t all doses tested were signi ficantly low er than those of veh icle-treated contr ols [15 mg/kg: F(1, 14) = 16.018, P b 0.001; 30 mg/k g:F(1, 14) =40.973,P b 0.001; 60 mg/kg: F(1, 14) = 4. 667, P = 0.049].Icariin-treat ed unstr essed subjects reveal ed a signifi cant re-duction in serum tT3 concent rations. The unstr essed anim als recei ving icariin treatmen t at 3 0 and 60 mg/kg signifi cantly reduced tT3 concent rations compa red to the vehicle contr ol group. It was n oted that there was no signifi cant difference between contr ol and stre ssed anim als recei ving a 60 mg/kg dose of icari in. Fluoxet ine show ed a slight decreas e in both stressed and unstressed groups compa red to the corres pondin g vehiclegroups (Fig. 6). 3.3.4. tT4 Serum t T4 concentrations (data not shown) did not show any d ifferenc es betwee n stressed a nd unstres sed control groups [ F (1, 14) = 0 .110, P = 0.745]. Se rum tT4 rema in ed u na ffe ct-ed by icariin treatments both in s tres sed [F (3, 28) = 0.447,P = 0 .7 21] and unstressed [F (3, 28) = 1.246, P = 0. 31 2 ]. Fl uo x-etine a lso e xhibited no e ff e c t (d at a n o t sh ow n) . 4. Discuss ion The presen t study was focuse d on the changes that CMS model induce d on a speci fic he donic deficit, the HPA axis and HPT axis. In addition, the influences of icariin and fluox etine treatmen ts on CMS-rel ated effects were examined. Exposure to 5 weeks of CMS produced a gradual decline in sucrose intake per unit body weight, relative to baseline sucrose intake and intake of the respective unstressed control group. The present result on sucrose consumption was in agreement to the findings of similar studies ( Willner et al., 1996; Li et al., 2003; Stathis et al., 2005; Gronli et al., 2005a,b). Noticeably, the sucrose consumption test is usually conducted by presenting the animals of a choice of solutions to drink during the test session –both water and the sucrose–and then the preference to chose sucrose over water is quantified. Simple tests of sucrose drinking may not be avalid measure of hedonic state when exposure to CMS in animals.Although water intake was not measured in the present study,some of recent studies have demonstrated that water intake was unaffected by the CMS procedure ( Grippo et al., 2002, 2004,2005b). In addition, neither CMS nor fluoxetine treatment af-fected water intake after 4 weeks of CMS (Grippo et al., 2006),suggesting that the changes in sucrose consumption might notbe accompanied by nonspecific changes in ingestive behavior,such as no change in water intake.Chr onic ad ministration of icari in in Wist ar rats treated with CMS procedu re, elicited a signi ficant rise in sucros e inta ke with a time–effect course. 3– 5 week s of icariin treat ment was required to revers e the defic it in sucros e consumpti on of CMS-treated rats. How ever, no signifi cant changes were observ ed in control animals treated with icariin. To our k nowledge, this is the first study that confi rms antide pressant-like acti vity of icariin in the CMS rats . A recent ly publi shed study demon strated that icariin treat ment reduced imm obility in the FST and TST in mice (Pa n et al., 2005). These beha vioral data mig ht confi rm previ ous clinical report s that E. brevicornumextra cts imp roved depres -sion symptom s after stroke (Lai, 2001; Ma, 2003). CM S induce d imp ortan t changes in the HPA a xis ( Azpiroz et al., 1999; Bratt et al., 2001; Kioukia -Fougia et al., 2002;Grippo et al., 2005a,b ). In the presen t study, the observ ation of the signifi cant CRF incre ases in dissected brain regio ns of the CMS-tre ated animals compa red with the contr ols was consi stent with the hypoth esis that brain CRF was up-reg ulat ed in major de p r e s s i o n (Nemeroff, 2000 ). Therefore t he central CRF systems in particula r might have a role in the respon se of rats to the CM S procedu re. The a bnormali ties in the HPA axis of CMS-tre ated rats might relat e to a hypersec retion of central CRF. In addition, incre ased CRF concent rations in serum were found in the CMS-treated rats, which ag reed with previ ous studies ( Widerlow et al., 1986; Catala n et al., 1998 ; Galard et al., 2002) that report ed incre ased plasma CRF concent rations in depressed disorders, but differed consi derably from anothe r work, which show ed unchange d neurope ptid e concent rations (Cha rlton et al., 1986). Althoug h general ly though t of as a brain CRF regulatin g pituitary – adrenal function, CRF immunor eac-tivity has been observ ed in a numbe r of perip heral tissues (Ow ens and Nem eroff, 1991). The hypoth alamic or peripheral origin of plasma CRF concent rations is controversi al. Since CRF reaches the pituita ry throu gh the hypoth alamo – hypophy-seal porta l circulation , alterations in PVN-C RF neural acti vity might be affected in the peripheral circu lation. CRF immuno-reactivity has a lso been observ ed in perip heral plasma of patients with HPA disorders (Su da et al., 1987; Cunnah et al., 1987; El lis et al., 19 90 ). At presen t, serum CRF level s incre ased in parallel to the CRF hypers ecret ion of hypoth alamic and other dissected brain regions in respon se to the CMS, sugges ting that peripheral CRF might be secreted mai nly co – by the hypothal-amus and extrah ypothalam us source s. On the other hand, CRF is the most potent ACTH secretagogu e. However, in the p resent study, the CMS procedu re render ed no relative difference s on serum ACTH concentrati ons in Wist ar rats , which was in line with ACTH concen trations found in Sprague– Dawle y rats exposed to CM S (Grippo et al., 2005b ). ACT H did not correl ate signifi cantly with CRF in CMS -treated rats. This dissociat ion between CRF an d ACTH o bserved by us and other author s ( Catala n et al., 1998; Pignatell i et al., 20 00; Ki oukia-F ougia et al., 2002) did not rule out a hypoth alam ic origin for perip heral CRF con centratio ns. In this study, the origi n of serum CRF was a confoun ding facto r in these deter minati ons, hence perip heral concent rations of CRF wer e difficult to inte rpret. Normal ization of the HPA axis syst em was hypoth esize d to play an importan t role for medi ating the anti depressant activity in pa tients ( Bschor et al., 2003; Ni ckel et al., 2003; Nikisch et al., 2005). In this study, we tested the hypoth esis that icari in admin istration would modul ate CRF in brain regio ns of rats. Our data elic ited that icariin was capabl e of attenua ting the CMS -induce d incre ases in CRF of different brain regio ns in rats, sugges ting centr al CRF system in stressed animals was sensitive to icariin treatmen t. The norm alization of elevat ed serum CRF concent rations in stre ssed animals treated with icariin was also observ ed. In addit ion, icariin adminis tration did not affect serum ACTH co ncentrati ons in stre ssed rats . Althoug h meas urement of CRF concent rations alone is insufficient to deter mine wheth er changes in synth esis, relea se or stor age or degrada tion , are respon sible, differences betwe en treat ment groups c learly rep-resent alterati ons in brain CRF system in funct ion of icari in. The fact that CRF changes accom panied with icariin-induc ed changes in behavi or durin g the CMS procedu re was interest ing. Taken toget her the above studies indicated that CRF appeared to play a role in the interactio n of CMS with icariin respon se, and activati on of brain CRF might be involved in the behavi oral cross -sensitizati on betw een CMS and icariin. Fluoxet ine reduced CRF in diss ected b rain regio ns of the CMS-tr eated rats without changing the serum CRF concent ra-tions. There were no effects of fluoxetin e adminis tration on basal , median eminence CRF concent ration, on depletion o f the peptid e after chroni c stress (Stout et al., 2002 ). It was plaus ible that a blunted effect of stress on CRF might contr ibute to no r-malizat ion o f the HPA axis activity b y fluox etine observ ed in the CMS-tr eated rats . In unstressed rats , icariin incre ased CRF both in serum and brain except in hypoth alam us, howe ver, these effects were partly dependen t on icariin doses tested. Fluoxet ine also produce d a rema rkably elevat ion of CRF in co rpus striatum and medul la oblong ata, ACTH concen trations in serum, whi ch were partly consi stent with an early report that fluox etine caused a sig-nificant increase in plasm a ACT H concent rations as wel l as in CRF concentrati ons of hypophys ial portal plasma (Gibbs and Vale, 198 3). In the presen t study, we also sought to charact erize stre ss regul ation of the HPT axis. We firstly demon strated that 5 week s of exposur e to the CM S procedu re caused a signifi cant decrease in serum tT3 concent rations, wi thout a signi ficant chan ge in tT4 concent rations in Wistar rats . These findings partly suppor ted previ ous studi es findi ngs that chronic stre ss to mice ( Cremas chi et al., 2000; Silbe rman et al., 2002 ), inesc apable foot-shock stress to Sprague – Dawle y rats caused a decreas e in perip heral thyro id horm one con centratio ns ( Helmr eich et al., 2005). It is well know n that brain CRF systems play a role in medi ating the neuroen docrine. Thyroid T3con tents were signific antly de-creased , but T 4 conten ts were not modified by CRF treat ment ( De Pedro et al., 1995), sugges ting that CRF suppre ssed thyroid functions (Tsigos and Chro usos, 2 002 ). Therefor e we speculated that the brain CRF might be more relev ant for regulatio n of thyroid horm ones and play a role on thyroid gland activity in the CMS rats . Furthe r evidenc e for regul atio n of the HPT axis durin g CMS comes from studies demon strating that gluoco cortico ids (corticos terone in rats) exert negati ve effects on the HPT ax is ( Morley, 1981). Stres s-induced corticost erone suppre ssed the peripheral convers ion of T4 to T3, resultin g in serum T3 decreas e ( Bianco et al., 1987). Al though corticost erone was not measured in the presen t study, most previous studies have demon strated th at CMS ca us ed an i nc re as e i n p la sm a co r t i co st er on e concent rations (Ayensu et al., 1995; Konk le et al., 2003; Grippo et al., 2005a,b ), indi cating that product of the HPA axis might induce serum tT 3 decreas e observ ed in stressed anim als. The negative correlation n of tT 3 to CRF in the CMS model of rats in this study is new. The observed pattern of alteration s in these Measures of the HPA axis and the HPT axis activities suggested That there were close interrelations between the HPA and the HPT axes, which were possibly affected during the CMS procedure. This area requires further investigation. In the present study, the failure of ocarina to rovers e CMS Effects on tT 3 Was observed. Icaria in showed a reduction in Serum tT3 Contents without any changes in tT 4, indicating that There was not a clearly closed interaction of ocarina treatment With thyroid function in the CM S rats. In an early study, small Decreases in plasma T 3 occurred after antidepressant desipramine administration for 14 days in rats (Atterwill et al., 1989). It was of considerabl e inte rest to noti ce that icariin altered serum tT 3 co nce ntra tions in uns tresse d and stre ssed an imals i n different patt ern. Thus, there may appear to be more complex in the thyroid horm one respon se to the HPA axis. As mentioned above, CRF suppre ssed the T 3 relea se, we quest ioned whether there was a negati ve correl ation between CRF and tT 3 in unstressed and stressed animals treated with different dosages of icariin. In unstressed anim als, signi ficant incre ases in CRF in cortex, hippoc ampus and corpus stri atum induce d by 30 mg/kg icariin treat ment, or in cortex and corpus striatum induce d by 60 mg/ kg icariin treat ment have been tigh tly correl ated to a signifi cant decreas e in serum tT 3 concent rations, respec tively. In stre ssed animals, 15 mg/ kg icariin- induced decreas ed CRF concent rations in hypothalam us seemed to be sligh tly lower than that induce d by 30 mg/ kg icariin treatmen t, while 15 mg/kg icariin- induced tT 3 concent rations was higher than that induc d by 30 mg/kg icariin treatmen t. Also in stre ssed anim als, the signifi cant decreas ed CRF c oncentrati ons (belo w the normal values) in cortex and corpus stri atum induce d by 60 mg/kg icariin treatmen t mig ht enhance the ability of the same dose of icariin to block CMS-in duced tT3 decrease, resultin g in no signifi cant reduction in T3 concentrati ons, which was in contr astto other findings in 15 and 30 mg/kg icariin- treated groups ,where similar reduct ions in tT3 and CRF in corte x and corpusstriatum wer e simultaneou sly found. These observ atio ns indicated that the neg ative correlati on betw een change s in some brain regio n CRF and serum tT 3concent rations appeared to be dependen t on the condition of the anima ls and icari in dosages . More speci fically, CRF in corte x and corpus striatum may be an importan t factor regul ating serum tT3 concentrati ons both in unstressed and stressed animals treated with 60 mg/ kg icari in. However, in some icariin- treated animals, there was no sig-nificant correlati on betw een brain CRF and serum tT3.Asan exam le, in contr ol anim als treated with 15 mg/kg icariin, CRF in corte x, hippoc ampus and corpus stri atum may not refl ect serum T3 con centratio ns, for no chang e in CRF or tT3 was observed. These findin gs indi cated that the neuroen docri nolog-ical effects of icariin might depend on the drug used, the durat ion of adminis tration and met hodolo gicalcriteria .Serum tT4 concent rations wer e unaffected in the stressed and unstressed anim als after fluox etine treat ment in this study. However, fluoxetin e showed a sli ght d ecrease bo th in stressed and unstr essed animals. Early report showed that acute admin-istration of fluoxetin e (2 and 10 mg/ kg ip) to no rmal rats elicited adecreas e on ly in T4 concent rations, whereas chroni c ad-minist ration resulted in a decreas e in both T3 and T4 concen-trations in rats (Golstein et al., 19 83 ). Rec ently, it was found that serum T4 but n ot T3 concent rations were signi ficantly lowered in normal rats after adminis tration of fluoxetin e at 15 mg/ kg for 14 day (Eravci et al., 2000). The imp act between unstressed and stressed anima ls treated with fluox etine was also observ ed when examining t he relatio nship between serum CR F and t T3. Althoug h in unstressed anim als, fluox etine treatmen t showed no significant changes in serum CRF and tT 3 concent rations,higher con centratio ns of CRF associated with lower concent ra-tions of tT3 were observ ed in stressed animals, sugges ting that there mig ht be in concord ance with negati ve regul ation of the HPA and the HPT axes in fluoxe tine-treated stressed anim als.In summary, we have found that a c hronic mild stress p ro-cedure generating an anhedoni c state in rats resulted in in-creased CRF concent rations in diss ected brain regio ns and serum, decreas ed tT 3 with no significant changes in serum ACTH and tT4 . This CMS provi ded a model to explor e mech-anisms of the HPA and HPT axes-medi ated events relev ant to human depression. Icariin reversed CM S-induced sucros e in-take reduct ion a nd CRF elevation. The se results sugges ted that the beh avioral profile of icari in acted predominan tly on the HPA axis system. In addition, the tT 3 changes in stressed and un-stressed animals indi cated a complex respon se to icariin treat-ment. Furthe r precl inical and clinical experi ments are required to clari fy the role o f icariin in contribut ing to treatmen t of depres sive disor ders. Acknowledgements The work was co-financ ed by grants from NSFC (Nos.30371755 and 90409009 ), NCE T-06-04 42 and JSNSF (B K2003070) to Ling-D ong Kong (L.D. Kong ). References Atterwill CK, Catto LC, Heal DJ, Holland CW, Dickens TA, Jones CA. Theeffects of desipramine (DMI) and electroconvulsive shock (ECS) on the function of the hypothalamo –pituitary–thyroid axis in the rat. Psychoneur-oendocrinology 1989;14:339 – 46. Ayensu WK, Pucilowski O, Mason GA, Overstreet DH, Rezvani AH, Janowsky DS. Effects of chronic mild stress on serum complement activity, saccharin preference, and corticosterone levels in Flinders lines of rats. Physiol Behav 1995;57:165–9. Azpiroz A, Fano E, Garmendia L, Arregi A, Cacho R, Beitia G, et al. Effects of chronic mild stress (CMS) and imipramine administration, on spleen mononuclear cell proliferative response, serum corticosterone level and brain norepinephrine content in male mice. Psychoneuroendocrinology 1999;24: 345–61 . Barden N. Implication of the hypothalamic –pituitary–adrenal axis in the physiopathology of depression. J Psychiatry Neurosci 2004;29:185 –93. Bauer M, Whybrow PC. Thyroid hormone, neural tissue and mood modulation. World J Biol Psychiatry 2001;2:59 – 69. Baumgartner A, Gräf KJ, Kürten I, Meinhold H, Scholz P. Neuroendocrino-logical investigations during sleep deprivation in depression. I. Early morning levels of thyrotropin, TH, cortisol, prolactin, LH, FSH, estradiol, and testosterone. Biol Psychiatry 1990;28:556 –68. Bianco AC, Nunes MT, Hell NS, Maciel RM. The role of glucocorticoids in the stress-induced reduction of extrathyroidal 3,5,3 ′-triiodothyronine generation in rats. Endocrinology 1987;120:1033–8. Bratt AM, Kelley SP, Knowles JP, Barrett J, Davis K, Davis M, et al. Long term modulation of the HPA axis by the hippocampus. Behavioral, biochemical and immunological endpoints in rats exposed to chronic mild stress. Psychoneuroendocrinology 2001;26:121–45. Bschor T, Baethge C, Adli M, Lewitzka U, Eichmann U, Bauer M. Hypothalamic –pituitary–thyroid system activity during lithium augmentation therapy in patients with unipolar major depression. J Psychiatry Neuosci 2003;28: 210–6. Cai D, Shen S, Chen X. Clinical and experimental research of Epimedium brevicornumin relieving neuroendocrino-immunological effect inhibited by exogenous glucocorticoid. Chin J Integr Med 1998;18:4 –7. Catalan R, Gallart JM, Castellanos JM, Galard R. Plasma corticotropin-releasing factor in depressive disorders. Biol Psychiatry 1998;44:15–20. Charlton BG, Leake A, Ferrier IN, Linton EA, Lowry PJ. Corticotropin-re-leasing factor in plasma of depressed patients and controls. Lancet 1986;1: 161–2. Cremaschi GA, Gorelik G, Klecha AJ, Lysionek AE, Genaro AM. Chronic stress influences the immune system through the thyroid axis. Life Sci 2000;67: 3171–9. Cunnah D, Jessop DS, Besser GM, Rees LH. Measurement of circulating corticotrophin-releasing factor in man. J Endocrinol 1987;113:123 –31. DeMoranville BM, Jackson IMD. Psychoneuroendocrinology. In: Fogel BS, Schiffer RB, Rao SM, editors. Neuropsychiatry. Baltimore, MD, USA:Williams A & Wilkins; 1996. p. 173–92. De Pedro N, Gancedo B, Alonso-Gomez AL, Delgado MJ, Alonso-Bedate M. Alterations in food intake and thyroid tissue content by corticotropin-releasing factor in Tinca tinca. Rev Esp Fisiol 1995;51:71 – 5. Ell is MJ , Sch mi dli RS , Do na ld RA, L ive se y JH , Esp in er EA . P la sma corticotrophin-releasing factor and vasopressin responses to hypoglycaemia in normal man. Clin Endocrinol 1990;32:93-100. Eravci M, Pinna G, Meinhold H, Baumgartner A. Effects of pharmacological and nonpharmacological treatments on thyroid hormone metabolism and concentrations in rat brain. Endocrinology 2000;141:1027–40. Fava M, Labbate LA, Abraham ME, Rosenbaum JF. Hypothyroidism and hyperthyroidism in major depression revisited. J Clin Psychiatry 1995;56:186 –92. Fountoulakis KN, Iacovides A, Grammaticos P, St Kaprinis G, Bech P. Thyroid function in clinical subtypes of major depression: an exploratory study. BMC Psychiatry 2004;4:6. Galard R, Catalan R, Castellanos JM, Gallart JM. Plasma corticotropin-releasing factor in depressed patients before and after the dexamethasone suppressiontest. Biol Psychiatry 2002;51:463 –8. Gibbs DM, Vale W. Effect of the serotonin reuptake inhibitor fluoxetine on corticotropin-releasing factor and vasopressin secretion into hypophysial portal blood. Brain Res 1983;280:176–9. Golstein J, Schreiber S, Velkeniers B, Vanhaelst L. Effect of fluoxetine, a serotonin reuptake inhibitor, on the pituitary–t h yr oi d a xi s i n r at . E ur J P ha rma col 1983;91:239 –43
Chlorogenic Acid corosolic acid Ursolic acid
HoneySuchle Flowers Extract Eucommia Leaf Extract Icariin
Amygdalin 10-hydroxycamptothecin Dihydromyricetin DMY
7-Ethyl-10-Hydroxycamptothecin Focus on plant extraction, especially proficient in extraction of high-purity natural products |







 Icariin from Epimedium brevicornum attenuates chronic mild stress-induced behavioral and neuroendocrinological alterations in male Wistar rats
Icariin from Epimedium brevicornum attenuates chronic mild stress-induced behavioral and neuroendocrinological alterations in male Wistar rats