| Icariin and differential protein expression in the entorhinal area of senescence-accelerated mouse prone 8 mice |
| 发布时间:2012-08-01 信息来源:admin 发布人:admin 点击次数:2672 |
|
Icariin and differential protein expression in the entorhinal area of senescence-accelerated mouse Abstract The present study sought to explore the mechanism of action by which icariin, an active component of Epimedii Herba, treats Alzheimer’s disease at the proteomics level. Two-dimensional gel electrophoresis was used to isolate total protein from the entorhinal cortex of senescence-accelerated mouse prone 8 (SAMP8) mice, and differential protein spots were obtained. Corresponding peptide mass fingerprinting was conducted through mass spectrography to identify differential protein spots. Twenty-six differential protein spots were found in the entorhinal area of SAMP8 mice at 8 weeks following intragastric perfusion with icariin and double distilled water. Fourteen spots were identified, which were involved in mitochondrial energy metabolism, oxidative stress, and neuronal function. The results revealed that icariin can regulate the expression of various proteins in the entorhinal cortex of SAMP8 mice, and treat Alzheimer’s disease by improving mitochondrial function, suppressing oxidative stress, inhibiting neural cell apoptosis, and protecting neurons. Key Words: icariin; Alzheimer’s disease; entorhinal cortex; proteomics; traditional Chinese medicine; neural regeneration INTRODUCTION The pathogenesis of Alzheimer’s disease (AD) remains controversial. AD is a complex disease caused by an interplay between multiple genetic and other factors[1-4]. In Chinese medicine, AD onset is characterized by renal deficiency. Recent studies have reported that icariin, a major constituent of the well known crude drug Epimedii Herba, can dilute senile plaques containing β-amyloid (Aβ), inhibit abnormal tau protein phosphorylation, suppress oxidative damage, reduce acetylcholinesterase (AChE) activity, and improve cognition of AD model rats[5-7]. We hypothesized that icariin may improve cognitive ability in AD model rats, via the regulation of Aβ metabolism, inhibition of abnormal tau protein phosphorylation, inhibition of oxidative damage and decreasing acetylcholinesterase activity. This prediction suggests that effective AD treatment involves multiple targets and pathways. The senescence-accelerated mouse prone 8 (SAMP8) model is an aging model characterized by homogeneous genetic background and stable features of senescence. SAMP8 mice exhibit Aβ deposition, periodic acid-Schiff positive particles, sponge-like pathological changes, and cognitive impairment, accompanied by rapid advancement of senescence after the growth period (4-6 months), which are similar to the progression of clinical AD[8]. SAMP8 mice represent an ideal model of brain aging and dementia, and have been extensively used in basic investigations of aging and nootropics. Previous results have demonstrated that entorhinal cortex atrophy precedes hippocampal atrophy in AD[9]. Characteristic pathological changes in AD, such as Aβ deposition and neurofibrillary tangles, occur early in the entorhinal cortex, and tangle density is positively correlated with dementia severity[10]. In the present study, SAMP8 mice were administered icariin, and differential protein expression in the entorhinal cortex was determined to elucidate the multi-targeted effects of icariin in treating AD.
RESULTS
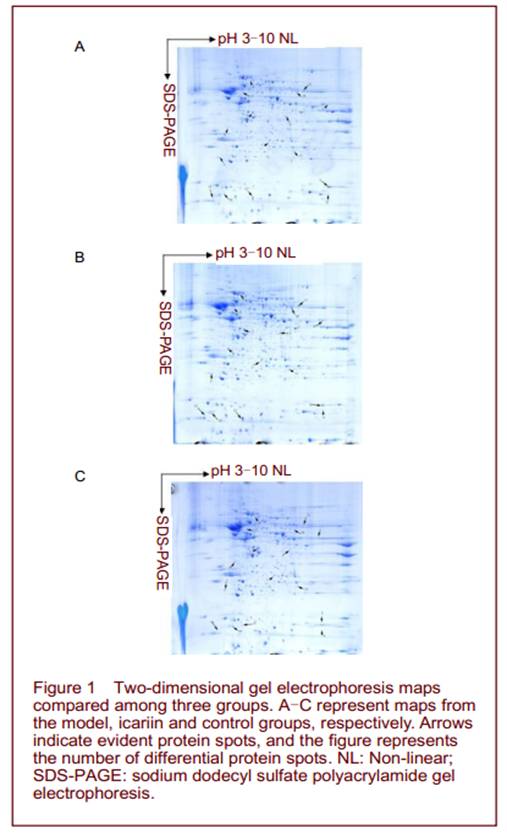
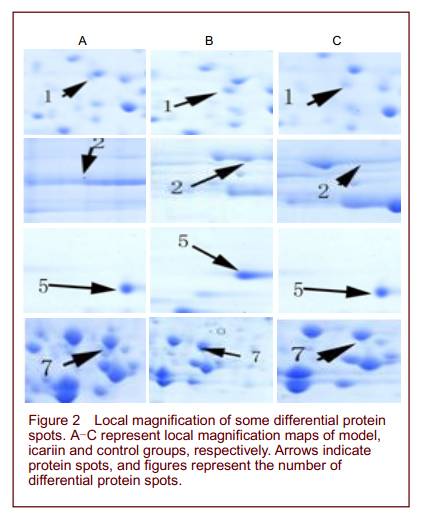
Quantitative analysis of experimental animals A total of 30 adult male SAMP8 mice were equally and randomly assigned to model, icariin and control groups. The model group was not treated. The icariin and control groups were intragastrically perfused with icariin and double distilled water, respectively. All 30 mice were included in the final analysis. Entorhinal two-dimensional gel electrophoresis (2-DE) maps and differential protein spot identification The sample volume of entorhinal area was 1 200 µg in each group. After sampling six times, six 2-DE maps with clear background, high resolution and repeatability were obtained from each group. PDQuest 7.0 analysis revealed 992, 1 022, 995 protein spots in the model, icariin and control groups, respectively, with mean matching rates of 88.1%, 87.3%, and 87.9%, respectively. Comparisons revealed 26 differential spots between the icariin and model groups, including 10 upregulated and 7 downregulated spots in the icariin group, 5 spots that were only expressed in icariin group, and 4 spots that were only expressed in the model group. Similar differences were found between the icariin and control groups, but no differences were detected between the model and control groups (Figures 1, 2).
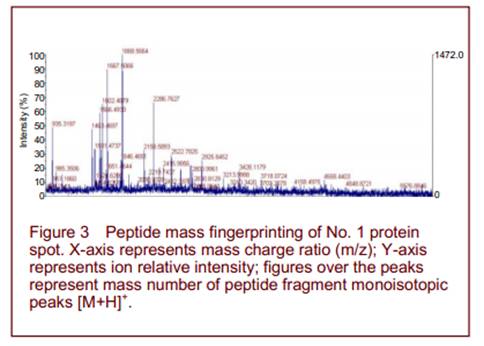
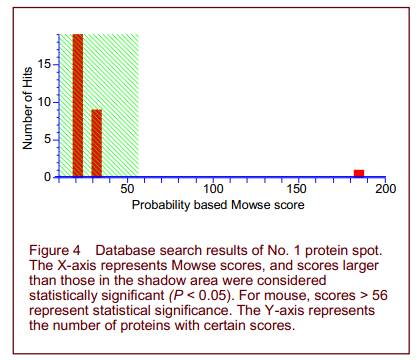
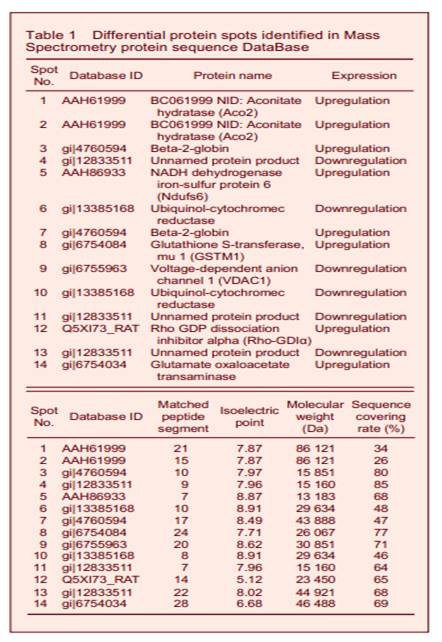
Peptide mass fingerprinting of protein spot No. 1 is shown in Figure 3. A total of 16 differential protein spots were selected for mass spectrography of differential protein spots. Searching the Mass Spectrometry protein sequence DataBase (MSDB) identified 14 of the spots (Figure 4 and Table 1). Spots 1, 2, 5, 9 were found to participate in mitochondrial energy metabolism, while spots 6, 8, 10, 12 were related to oxidative stress. Spots 3, 7, 14 exerted protective effects on neurons. Spots 4, 11, 13 were unknown proteins. Spots 1 and 2, 3 and 7, 6 and 10 were found to be the same protein.
DISCUSSION
Mitochondrial energy dysmetabolism is highly correlated with AD, and metabolic decrease is related to the severity of dementia[11]. Aco2 and Ndufs6 are subunits of nicotinamide adenine dinucleotide dehydrogenase, and participate in enzyme reactions involved in mitochondrial oxygen exchange. In addition, voltage-dependent anion-selective channel protein 1 (VDAC1) overexpression can induce mitochondrial energy dysmetabolism[12]. In the present study, icariin was found to upregulate Aco2 and Ndufs6, and downregulate VDAC1 expression. It is likely that icariin enhances mitochondrial enzyme activity, improving mitochondrial energy metabolism and increasing brain function to treat AD.
Oxidative stress represents an imbalance between the production and manifestation of reactive oxygen species and a biological systems ability to readily detoxify the reactive intermediates or repair the resulting damage. Free radical-induced cell damage constitutes the major pathogenesis of AD[13]. Ubiquinol-cytochromec reductase is the main constituent involved in producing superoxide and hydrogen peroxide, and is responsible for the mitochondrial electron transport chain that produces active oxygen-derived free radicals. Rho protein can induce oxidative stress[14], and Rho-GDIα negatively regulates Rho protein activity. Glutathione S-transferase Mu 1 is involved in an important detoxifying system during anti-oxidative damage and free radical clearance[15-16]. In the current study, icariin was found to decrease ubiquinol-cytochrome c reductase, and increase Rho-GDIα and Glutathione S-transferase Mu 1 expression for anti-oxidation and aging to treat AD. GOT and beta-2-globin can improve the oxygen-carrying capacity of tissues and benefit neuronal function[17]. In the present study, icariin increased GOT and beta-2-globin expression. Therefore, we propose that icariin improves AD symptoms by improving neuronal energy metabolism and protecting neurons. Proteomics has been used to study protein changes in complex diseases. However, there are some limitations that must be considered. Some low-abundance proteins with strong bioactivities play essential roles in AD onsetand treatment, and proteomics cannot accurately isolate or identify low-abundance proteins. Therefore, potential target proteins that directly influence treatment will not be isolated or identified. The replicability of proteomics remains problematic. Although large-scale proteomic studies have been conducted in some diseases, the results for single diseases often vary between studies. This discrepancy is likely to be due to the low level of automatic operations in time consuming proteomics analyses, and to false positive results related to human factors. Therefore, the results of proteomics studies require further verification from other types of research. In conclusion, our results indicate that icariin can regulate the expression of various proteins in the entorhinal cortex of SAMP8 mice, and treat AD by improving mitochondrial function, suppressing oxidative stress, inhibiting neural cell apoptosis, and protecting neurons.
MATERIALS AND METHODS
Design A randomized, controlled, proteomics animal experiment. Time and setting This experiment was performed at the Key Laboratory of Cancer Proteomics of Chinese Ministry of Health, Xiangya Hospital, Central South University, China from February to October 2009. Materials Thirty male SAMP8 mice, aged 6 months, weighing 18-22 g, were purchased from the Department of Animals, First Hospital of Tianjin University of Traditional Chinese Medicine. The mice were fed with steam-sterilized water and food in a laminar flow room at 18-22°C and 55-58% humidity. Icariin (purity 98%), light yellow crystal, was purified in the Department of Biochemistry, Xiangya Medical School of Central South University, China. The chemical structural formula was C33H40O15 . Methods Drug administration Icariin group was intragastrically administrated icariin (suspended with 0.5% sodium carboxymethylcellulose) for a volume of 0.01 mL/g body mass; the control group was intragastrically administered the same volume of double distilled water, once daily for 8 consecutive weeks. Sample collection and treatment Mice in three groups were anesthetized and sacrificed after 8 weeks. The entire brain tissues were removed and washed with cold normal saline. Tissues in bilateral entorhinal cortices were harvested on ice[18], washed with cold normal saline, packaged in a frozen tube, and stored in liquid nitrogen. Total protein extraction and concentration determination The entorhinal cortical tissues were harvested from liquid nitrogen, placed in EP tubes of known weight, and weighed, mixed with tissue lysate (50 mg sample: 400 µL lysate), ground using a sample grinding kit (Amersham Biosciences, Buckinghamshire, UK), incubated at room temperature for 1 hour, and centrifuged at 12 000 r/min at 4°C for 1 hour. The supernatant was collected, i.e. total protein. 5 µL supernatant was used to determine protein concentration with a 2D Quant Kit (Amersham Biosciences). The remaining supernatant was packaged and stored at -70°C. 2-DE 2-DE was performed according to the instructions of an IPGphor isoelectric focusing system (Amersham Biosciences), and a previously described method[19]. Briefly, freshly prepared Coomassie brilliant blue G-250 staining solution (Amersham Biosciences) was placed on a gel-coated disk, 250 mL for each piece of gel, and completely covered the gel, overnight on a shaking table, washed with double-distilled water for twice after discarding the staining solution, mixed with freshly prepared destaining solution (250 mL for each piece of gel), and placed on a shaking table for 15 minutes. The destaining solution was discarded, and double distilled water was added to cover the gel. The gel was scanned using an Imagescanner system (Imagescanner II, Bio-Rad, Hercules, CA, USA). Spot detection, background subtraction, matching, and quantization were performed using PDQuest 7.0 image analysis software (Bio-Rad) to acquire spot information. Mass spectroscopy Protein spots were excised from the gel and placed in 1.5-mL Eppendorf tubes. Mass spectrum samples were prepared according to a previously described method[20]. The samples were analyzed using Voyager-DE STR 4307 MALDI-TOF-MS (Applied Biosystems, Carlsbad, CA, USA) according to previously described method[20]. Using Mascot software (Matrix Science, London, UK), a *.DAT document containing peptide mass fingerprint data was input for computer-based online search of http://www.matrixscience.com. MSDB and NCBInr databases were also searched.
Author contributions: Ting Zhang conceived and designed this study, analyzed data and wrote the draft of the manuscript. Zhanwei Zhang was in charge of data integration and support. Keli Dong authorized the manuscript and guided the study. Guangcheng Li provided technical support. Hong Zhu analyzed the data. Conflicts of interest: None declared. Ethical approval: This study received permission from the Animal Ethics Committee of Central South University, China. Acknowledgments: We thank Zhiqiang Xiao, Fang Peng, and Cui Li, Laboratory of Tumor Proteomics, Xiangya Hospital, and Xiaowei Xing, Central Laboratory of Third Xiangya Hospital, China for technical support. The authors also thank Yan Chen, Laboratory of Tumor Proteomics, Xiangya Hospital for the help. Supplementary information: Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org, and entering Vol. 6, No. 18, 2011 after selecting the “NRR Current Issue” button on the page.
REFERENCES
[1] Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimers disease. Nature. 2009;461(7266):916-922. [2] Palop JJ, Chin J, Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006;443:768-773. [3] Noorbakhsh F, Overal CM, Power C. Deciphering complex mechanisms in neurodegenerative diseases: the advent of systems biology. Trends Neurosci. 2009;32(2):88-100. [4] Villoslada P, Steinman L, Baranzini SE. Systems biology and its application to the understanding of neurological diseases. Ann Neurol. 2009;65(2):124-39. [5] Urano T, Tohda C. Icariin improves memory impairment in Alzheimers disease model mice (5xFAD) and attenuates amyloid β-induced neurite atrophy. Phytother Res. 2010;24(11):1658-1663. [6] Zeng KW, Ko H, Yang HO, et al. Icariin attenuates β-amyloid-induced neurotoxicity by inhibition of tau protein hyperphosphorylation in PC12 cells. Neuropharmacology. Zhang T, et al. / Neural Regeneration Research. 2011;6(18):1370-1374. 1374 2010;59(6):542-550. [7] He XL, Zhou WQ, Bi MG, et al. Neuroprotective effects of icariin on memory impairment and neurochemical deficits in senescence-accelerated mouse prone 8 (SAMP8) mice. Brain Res. 2010;1334:73-83. [8] Nomura Y, Okuma Y. Age-related defects in lifespan and learning ability in SAMP8 mice. Neurobiol Aging. 1999;20(2):111-115. [9] Pennanen C, Kivipelto M, Tuomainen S, et al. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol Aging. 2004;25(3):303-310. [10] Carr DB, Goate A, Phil D, et al. Current concepts in the pathogenesis of Alzheimer’s disease. Am J Med. 1997; 103(3A):3S-10. [11] Drzezga A, Riemenschneider M, Strassner B, et al. Cerebral glucose metabolism in patients with AD and different APOE genotypes. Neurology. 2005;64(1):102-107. [12] Abu-Hamad S, Sivan S, Shoshan-Barmatz V. The expression level of the voltage-dependent anion channel controls life and death of the cell. Proc Natl Acad Sci U S A. 2006;103(15): 5787-5792. [13] Christen Y. Oxidative stress and Alzheimer disease. Am J Clin Nutr. 2000;71(2):621S-629S. [14] Bokoch GM, Diebold BA. Current molecular models for NADPH oxidase regulation by Rac GTPase. Blood. 2002;100(8): 2692-2696. [15] Rosario LA, OBrien ML, Henderson CJ, et al. Cellular response to a glutathione S-transferase P1-1 activated prodrug. Mol Pharmacol. 2000;58(1):167-174. [16] Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30(6):445-600. [17] Burmester T, Weich B, Reinhardt S, et al. A vertebrate globin expressed in the brain. Nature. 2000;407(6803):520-523. [18] Paxinos G., Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press. 1982. [19] Görg A, Obermaier C, Boguth G, et al. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis. 2000;21(6):1037-1053. [20] Li C, Zhan X, Li M, et al. Proteomic comparison of two-dimensional gel electrophoresis profiles from human lung squamous carcinoma and normal bronchial epithelial tissues. Genomics Proteomics Bioinformatics. 2003;1(1):58-67.
Chlorogenic Acid corosolic acid Ursolic acid
HoneySuchle Flowers Extract Eucommia Leaf Extract Icariin
Amygdalin 10-hydroxycamptothecin Dihydromyricetin DMY
|







 Icariin and differential protein expression in the entorhinal area of senescence-accelerated mouse prone 8 mice
Icariin and differential protein expression in the entorhinal area of senescence-accelerated mouse prone 8 mice