| Comparative pharmacokinetics of icariin in plasma after oral administration of Er-Xian decoction or pure icariin in rats |
| 发布时间:2012-07-31 信息来源:admin 发布人:admin 点击次数:4233 |
|
Comparative pharmacokinetics of icariin in plasma after oral administration of Er-Xian decoction or pure icariin in rats The present study was to investigate the pharmacokinetics of icariin in rat after oral administration of single dose of Er-xian decoction (EXD) and pure icariin. Blood samples were collected by orbital vein at time intervals after drug administration and the plasma concentrations of the studied components were determined by high performance liquid chromatography (HPLC) after the plasma protein was precipitated directly with acetonitrile. Icariin was successfully separated using a C18 column with a UV detection at 270 nm and a mobile phase of acetonitrile–water with 0.1% H3PO4 (35:65, v/v) pumped at 1.0 ml/min. The method had a lower limit of quantitation (LOQ) of 0.60 μg/ml for icariin with the limit of detection (LOD) 0.35 μg/ml, based on a signal-to-noise ratio of 3. The assay was linear over a range 0.667 to 42.67 μg/ml with coefficient of determination greater than 0.99 for analytes. The extraction recoveries was >80%. The method has shown tremendous reproducibility, with intra- and inter-day precision <4.9% (RSD), and has proved to be highly reliable for the analysis of plasma samples. The main pharmacokinetic parameters of icariin in rats after administration, EXD and icariin were processed by Das 2.0, while calculating the pharmacokinetic parameters of one compartment model. From the experimental results, the method had been applied successfully to pharmacokinetics of icariin in rat plasma after oral administration of pure icariin or EXD.
Key words: Icariin, Er-xian decoction, pharmacokinetics. INTRODUCTION
Er-Xian decoction (EXD), a traditional Chinese medicine formula comprised of Curculi Rhizoma, Epimedii Folium, Morindae Officinalis Radix, Anemarrhenae Rhizoma, Phellodendri Cortex and Angelicae sinensis Radix, has long been used widely for the treatment of osteoporosis disorders (Wang et al., 1998), menopausal syndrome (Liu et al., 2005), and aging diseases for several decades (Shen et al., 1995). We previously have reported that EXD has positive effects on bone in ovariectomized rats (Nian et al., 2006), the flavonoids, saponins, alkaloids and anthraquinines present in EXD are responsible for its antiosteoporotic activity (Qin et al., 2008), stimulate osteoblastic UMR-106 cell proliferation, decrease tartrate-resistant acid phosphatase (TRAP) activity and bone resorption of osteoclasts derived from RAW264.7 cells. Proteomic investigate indicated that EXD modulates bone metabolism through regulation of osteoblastic proliferation, apoptosis, cell activation, osteoclastic protein folding and aggregation (Zhu et al., 2010). Icariin is one of the most important bioactive ingredients extracted from Epimedii Folium, which have the biological activities on promotion of bone formation (Wei et al., 2011), improvement of the learning and memory abilities (Nie et al., 2010; Guo et al., 2010), induction of osteoblasts proliferation and differentiation, resulting in bone formation (Hsieh et al., 2010). Pharmacokinetic study of active ingredients in natural product and Chinese herbs is important to illustrate their action mechanism for the development of traditional Chinese medicine (Ma et al., 2007). Pharmacokinetics studies on icariin (Cheng et al., 2007; Zou et al., 2002) in some herb extracts have been carried out. However, the pharmacokinetics of icariin in EXD has not been studied further. The high performance liquid chromatography (HPLC) method with high specificity and sensitivity has been employed to determine icariin in various extraction of icariin. However, method for determination of icariin in plasma after administration of EXD has not been reported. It is necessary to develop an analytical method for its determination in biological samples, for example, plasma. In this paper, a simple, rapid, sensitive and accurate method was applied to determine the plasma levels of icariin and has been successfully applied to pharmacokinetic investigation after the oral administration of icariin and EXD. In this study, a simple, rapid, accurate and reliable method was established for determination of icariin in rat plasma. The author had comparative evaluated the pharmacokinetics of icariin in rats after oral administration of EXD which have been used to treat osteoporosis for years, or pure icariin. The pharmacokinetics parameters were analyzed by software DAS 2.0 (Chinese Pharmacological Society, China). Differences among pharmacokinetics of icariin after oral administration of EXD or pure icariin in rats are illustrated. It is good for answering the combination of EXD and clinical research of icariin in osteoporosis. The validated method is successfully applied to control the quality of icariin and investigate inter action among other herbals of EXD in osteoporosis disorders and menopausal syndrome.
MATERIALS AND METHODS
Chemicals and reagents
Preparation of EXD consists of six plant materials, including Curculi Rhizoma, Epimedii Folium, Morindae Officinalis Radix, Anemarrhenae Rhizoma, Phellodendri Cortex and Angelicae sinensis Radix,in a compositional ratio of 12:12:9:9:10:10. The aforementioned six medicinal materials were obtained from Hua Yu Pharmaceutical Company and identified by Professor H.C. Zheng of the Department of Pharmacognosy, School of Pharmacy of the Second Military Medical University, Shanghai, China. Voucher specimens (numbered as 2007091901; 2007091902; 2007091903; 2007091904; 2007091905 and 2007091906, respectively) are available at the herbarium of this department. The six medicinal materials in the mixture (250 g) were powdered and extracted with distilled water [3000 ml, 12:1 (v/w)] at 100°C for 2 h. The extraction was repeated twice. The filtrates were concentrated to 1.5 L under reduced pressure, and lyophilized in a freeze drier, and kept at 4°C for quality control and molecular studies. The yield of dried extract from the starting crude materials was 17.8%. Icariin and benzoic acid was purchased from National Institute for The Control of Pharmaceutical and Biological Products (Beijing, China). Methanol and acetonitrile (all of HPLC grade) were provided by Fisher Scientific International, Inc. (Fair Lawn, NJ, USA). Purified water was prepared in a water purification system (Mili-Q Biocel., USA). All other reagents were analytical grade and purchased from Tianjin Kermel Chemical Reagents Co. Ltd. (Tianjin, China).
Chromatographic conditions
The reversed phase-high performance liquid chromatographic (RP-HPLC) analysis was carried out on an Agilent high performance liquid chromatography system (series 1200, Agilent Technology, Palo Alto, CA, USA) including a quaternary pump, vacuum degasser, thermostatic column compartment and a diode array detector (DAD). The chromatography data were recorded and processed with Agilent chemstation software. An Agilent Extend C-18 analytical column (5 μm, 4.6 × 250 mm) was used. The mobile phase consisted of acetonitrile and water with 0.1% H3PO4 in volumetric ratio of 35/65. The injection volume was 20 μl. The flow rate was set to 1.0 ml/min throughout the elution, and the temperature was set to 30°C and wavelength to 270 nm.
Preparation of stock solutions
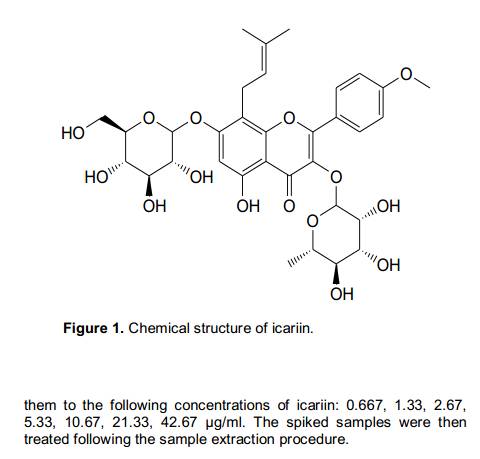
Accurately weighed 10.06 mg of standard sample icariin (structure shown in Figure 1) was transferred to a 10 ml volumetric flask which was then filled to mark with methanol. Final concentration of the resulted stock solution was 1.006 mg/ml. Standard curve solutions were freshly prepared based on diluting the above stock solution with methanol at appropriate ratios to yield concentrations of 2.00, 4.00, 8.00, 16.01, 31.02, 64.04, 128.08 μg/ml of icariin. The stock solution and working standard solutions consisting of icariin were stored at -4 and 4°C, respectively. Internal standard stock solution of benzoic acid (0.313 mg/ml) was also prepared in methanol and diluted with methanol for a working solution of 3.13 μg/ml.
Extraction procedure
An aliquot of 0.8 ml acetonitrile with 0.1 ml hydrochloric acid was added to 150 μl plasma sample to precipitate proteins with 50 μl internal standard in a centrifuge tube and the mixture was vortexed for 3 min. After centrifuged at 10000 rpm for 5 min, the supernatant was transferred into another tuber and evaporated to dryness at 35°C. The residue was dissolved by 50 μl methanol.
HPLC validation procedures
Calibration curves and linearity
The standard calibration curves in plasma were prepared daily by spiking the blank rat plasma (150 μl) with 50 μl of the standard solutions and 50 μl internal standard prepared earlier and making
Precision, accuracy and recovery
The intra-day accuracy and precision of five replicates quality control samples were determined within one day at concentrations of 1.33, 21.7 and 36.3 μg/ml for icariin. The inter-day accuracy and precision of the quality control samples were determined on three different days within one week. The relative standard deviation (%, RSD) of each concentration was calculated to determine the precision of the method. The recovery included extraction recovery and assay recovery. The extraction recovery was determined for three different concentrations of icariin at 1.33, 21.7 and 36.3 μg/ml by comparing the peak area ratio of icariin to I.S in plasma with that in methanol at same concentration. The assay recovery was determined in the same method, but calculated by comparing the concentrations of the analyte from the calibration curves with that in the prepared sample. The mean assay recovery value (%, mean±S.D) represented the accuracy of the method.
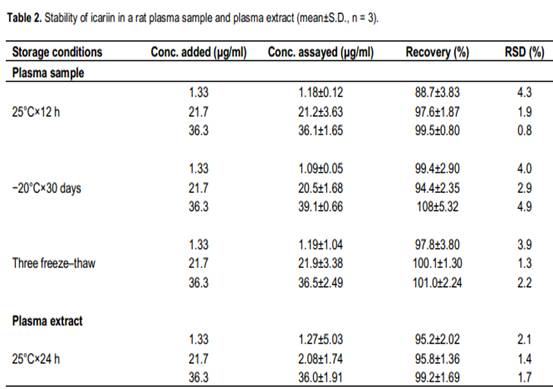
Stability
The quality control plasma samples in triplicates at low, medium and high concentration were used, which were prepared by spiking the blank rat plasma (150 μl) with 50 μl of the appropriate icariin solution and mixing them to yield the following concentrations 1.33, 21.7 and 36.3 μg/ml. Short-term temperature stability was assessed at room temperature (25°C) for 12 h. Long-term stability evaluations involved an analysis of quality control samples that were stored at -20°C for 30 days. Freeze-thaw stability was evaluated after three cycles of thawing at room temperature followed by re-freezing to –20°C. Post-preparation stability was also determined using the extracted samples that were kept at room temperature for 24 h prior to injection and analysis by HPLC.
Applicability of the method in pharmacokinetic studies
Animals
Ke Experimental Animal Ltd. (Shanghai China), n = 12, weighing 180±10 g. All these animals were housed in an air-conditioned room (temperature, 25°C; relative humidity, 55%), and allowed to freely access to food and water. The rats were fasted for 12 h before dosing.
Pharmacokinetics
The rats were divided into two groups (six rats per group), respectively. The dosing icariin and EXD were all dissolved in water. One group was assigned to receive icariin solution by oral administration at a single dose of 4.5 mg/kg of icariin, the other group was assigned to receive 4.8 g/kg of EXD solution. Serial blood samples (0.5 ml) were obtained via the rats orbital vein at 0.25, 0.5, 1, 2, 4, 6, 8, 12, 24 h after administration and collected into heparinized centrifuge tubes. The blood samples were immediately centrifuged at 4000 g for 5 min at room temperature. The plasma sample was extracted and analyzed according to extraction procedure. The data analysis of plasma concentration of icariin versus time and calculation of pharmacokinetic parameters in rats were executed by the statistics software DAS 2.0. The pharmacokinetic parameters were estimated using compartmental and statistical moment (that is, compartmental) analysis methods.
RESULTS AND DISCUSSION
Specificity
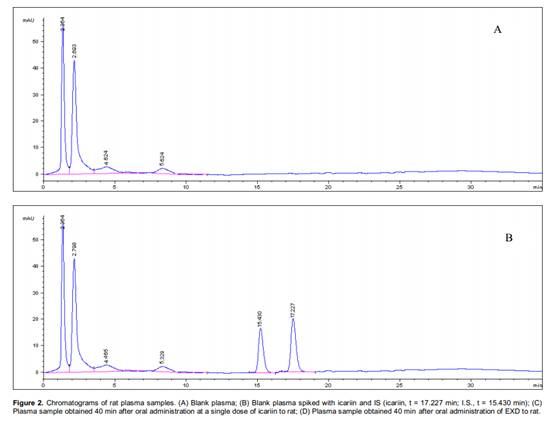
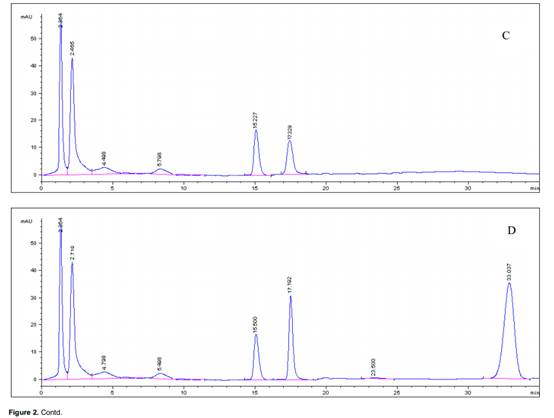
Typical chromatograms obtained from blank plasma, spiked rat plasma and plasma samples were shown in Figure 2. The peaks of the analyte in the plasma were identified by comparing their retention time with that of the standard sample. In this study, the retention times of icariin varied from 17.19 to 17.22 min. The specificity of the assay was investigated by injecting blank plasma. No peaks were observed that would interfere with the analysis in HPLC chromatograph. In this study, several organic solvents were tested for precipitating protein and extracting the analyte from rat plasma. They included methanol with 0.1 ml hydrochloric acid, acetonitrile with 0.1 ml hydrochloric acid and methanol-ethyl acetate (1:1, v/v) and their volume were one to four times that of plasma sample. The result showed that the effect of precipitating protein and extraction recoveries were best when acetonitrile with 0.1 ml hydrochloric acid was chosen. The extraction recoveries of the analyte at low, middle and high were 86.2±1.58%, 85.8±1.80% and 89.65±0.20%, respectively. In addition, 10% salicylsulfonic acid was used to precipitate protein in the experiment. It has a conspicuous effect on precipitating protein, but defects of which showed a bad peak shape.
Linearity
The peak areas of the analyte were plotted against standard concentrations to establish calibration curves for icariin. The curves were best fitted using a least square
linear regression model y = mx+b, weighted by 1/x2, in which y is the peak area ratio, m is slope of the calibration curve, b is intercept of the calibration curve and x is the analyte concentration. The slopes of the calibration graphs were 0.138±0.29 (n = 6) with RSD of 1.68%, and the intercepts were -0.0470±0.20 (n = 6) with RSD of 2.20% throughout the study. The correlation coefficients (r > 0.99) showed good linearities. This result showed the usefulness of the present HPLC method in the assays of icariin from low to high plasma levels. The limit of quantitation (LOQ) was achieved as the lowest point on the standard curve, 0.60 μg/ml for icariin with RSD of 5.8% (n = 5). The limit of detection (LOD) was defined as the lowest concentration of the drug resulting in a signal-to-noise ratio of 3:1. The limit of detection (LOD) of the analyte in plasma was determined to be 0.35 μg/ml for icariin.
Precision, accuracy and recovery
Quality control samples (n = 5) representing low, medium and high concentration contained 1.33, 21.7 and 36.3 μg/ml for icariin. The maximum intra- and inter-day precision was 4.9% for icariin. The assay showed that the intra- and inter-day precisions for icariin with a RSD of less than 15% were obtained at the lowest concentrations. Data on accuracy about the recovery of icariin was 86.2±1.58% (n = 5) at the lowest concentration of 1.33 μg/ml in spiked rat plasma samples (Table 1). The mean extraction recovery of the analyte (n = 5) from spiked rat plasma (Table 1) was satisfactory at low, medium and high concentrations, which varied from 80.4±3.97 to 89.7±0.20%. High recovery of icariin from rat plasma suggested that there was negligible loss during drug extraction.
Stability
Acceptable analyte stability was demonstrated for all phases of storage and processing was shown in Table 2. The accuracy of low (1.33 μg/ml), medium (21.7 μg/ml) and high (36.3 μg/ml) concentrations of icariin in rat plasma for 12 h room temperature stability (88 and 98%), three cycle freeze-thaw stability (94 and 108%), 30 days at -20°C, stability (97 and 101%) and 24 h post-preparative stability (95 and 100%) were all acceptable. The RSD (%) for each of the stability experiments varied between 0.9 and 4.9%. Application of the HPLC method for pharmacokinetic studies
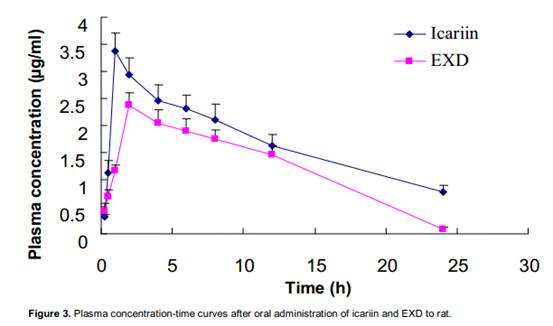
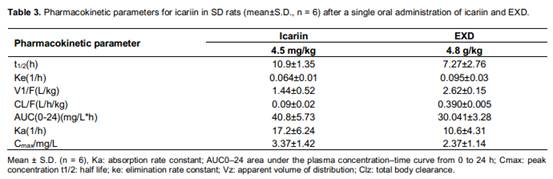
The debris assessment software (DAS) program as validated methods has been successfully applied for pharmacokinetic studies of icariin in rat plasma after oral administration of an icariin solution. The mean plasma concentration versus time profiles of icariin was shown in Figure 3. The pharmacokinetic parameters were listed in Table 3. With minimum akaike information criterion (AIC) values, a one-compartment open pharmacokinetic model was proposed. Icariin appeared to be distributed most rapidly into a highly perfused central compartment. The phenomenon with a rapid distribution and a relatively slow distribution followed by a slower elimination phase was observed from the compartment model parameters t1/2. The values of t1/2, ke, V1, CL, ka and Cmax of icariin in rat plasma.
Statistical analysis
The author had compared pharmacokinetics of icariin between pure icariin and Er-Xian decoction. The differences were showed in two aspects: the absorption peak of iacriin and absorption amount. It was noted that other ingredients among EXD
influenced the pharmacokinetics of icariin. As shown in Figure 3 after administration of pure Icariin, the absorption peak was larger than administration of EXD, and absorption amount AUC0−t: (40.8±5.73) vs. (30.04±3.28) (mg/L)· h in rat. The similar absorption result had happened in Pengs study (Peng et al., 2010). The author analyzed that the reasons were enterohepatic circulation and effect of other herbs. Under the influence of enterohepatic circulation, icariin had been already metabolized which would excrete and re-absorbed. In addition to enterohepatic circulation, the other reason was that there were interactions among different herbs. EXD was a combination of Curculi Rhizoma, Epimedii Folium, Morindae Officinalis Radix, Anemarrhenae Rhizoma, Phellodendri Cortex and Angelicae sinensis Radix, each of which could produce certain
pharmacological effects, and may have impacts on the absorption and metabolism of icariin. The author suggests that Curculi Rhizoma, Anemarrhenae Rhizoma and Phellodendri Cortex had antiosteoporotic activity and would block the enterohepatic circulation. Thereby they could weaken the absorption peak of icariin. It could surmise that multi-componet in EXD and their interactions resulted in the difference of the pharmacokinetics of EXD and pure icariin.
Conclusions
In conclusion, a simple HPLC assay method has been used for determination of icariin in rat plasma with an internal standard. Treatment with acetonitrile and hydrochloric acid for icariin results in chromatograms free of interference. The procedures for the determination of icariin by HPLC method have been validated, and the results demonstrate that the standard curve is linear over the concentration of 0.667 to 42.67 μg/ml. 150 μl plasma was required for analysis of the compound. The method is suitable for pharmacokinetic study of icariin in rats after oral administration. In summary, results of the present study indicated that disease and other herbs in decoction influenced the potential pharmacokinetic of icarrin.
ACKNOWLEDGEMENT
This work was supported by the National Natural Science Foundation of China, no. 90709023.
REFERENCES
Cheng S, Qiu F, Wang S, He J (2007). HPLC analysis and pharmacokinetics of icariin in rats. J. Sep. Sci., 30(9): 1307-12. Guo FL, Wu Q, Gong Q, Lu Y, Shi J (2010). Protective effects of icariin on brain dysfunction induced by lipopolysaccharide in rats. Phytomedicine, 17(12): 950-955. Hsieh TP, Sheu SY, Sun JS, Chen MH, Liu MH (2010). Icariin isolated from Epimedium pubescens regulates osteoblasts anabolism through BMP-2, SMAD4, and Cbfa1 expression. Phytomedicine, 17(6): 414-423. Liu Q, Shi JR, Yang Y, Fang ZQ, Liang SH, Guo RX (2005). Effects on secretory function of rat gonad by Er-Xian Decoction and its disassembled prescriptions. China J. Chin. Mater. Medica., 30: 1023-1026. Ma G, Jiang XH, Chen Z, Ren J, Li CR, Liu TM (2007). Simultaneous determination of vitexin-4-O-glucoside and vitexin-2-O-rhamnoside from hawthorn leaves flavonoids in rat plasma by HPLC method and its application to pharmacokinetic studies. J. Pharm. Biomed. Anal., 44: 243-249. Nian H, Qin LP, Zhang QY, Zheng HC, Yu Y, Huang BK (2006). Antiosteoporotic activity of Er-Xian Decoction, a traditional Chinese herbal formula, in ovariectomized rats. J. Ethnopharmacol., 108: 96-102. Nie J, Luo Y, Huang XN, Gong QH, Wu Q, Shi JS (2010). Icariin inhibits beta-amyloid peptide segment 25–35 induced expression of β-secretase in rat hippocampus. Eur. J. Pharmacol., 626(2-3): 213-218. Qin LP, Han T, Zhang QY, Cao DP, Nian H, Rahman K, Zheng HC (2008). Antiosteoporotic chemical constituents from Er-Xian Decoction, a traditional Chinese herbal formula. J. Ethnopharmacol., 118: 271-279. Shen XH, Fang ZQ, Wu DX (1995). Effect of Er-Xian Decoction and its disassembled prescription on enzyme activity and their gene expression of antioxidant enzymes in aging rat. Chin. J. Integr. Trad. Western Med., 15: 672-674. Wang CH, Zhang ZH, Ma J (1998). Treatment of 50 cases of osteoporosis with Er-Xian Decoction. Shan Xi Zhong Yi, 19: 205. Wei H, Zili L, Yuanlu C, Biao Y, Cheng L, Xiaoxia W, Yang L, Xing W (2010). Effect of icariin on bone formation during distraction osteogenesis in the rabbit mandible. Int. J. Oral Maxillofac Surg., 40(4): 413-418. Zhu Z, Xue LM, Han T, Jiao L, Qin LP, Li YS, Zheng HC, Zhang QY (2010). Antiosteoporotic Effects and Proteomic Characterization of the Target and Mechanism of an Er-Xian Decoction on Osteoblastic UMR-106 and Osteoclasts Induced From RAW264.7. Molecules, 15(7): 4695-4710. Zou JM, Meng J, Yan ZH, Long ZX, Shi XJ (2002). Pharmacokinetic studies of icariin in Chinese formulated medicine. Chin. Trad. Herbal Drugs, 33(1): 55.
Chlorogenic Acid corosolic acid Ursolic acid HoneySuchle Flowers Extract Eucommia Leaf Extract Icariin |







 Comparative pharmacokinetics of icariin in plasma after oral administration of Er-Xian decoction or pure icariin in rats
Comparative pharmacokinetics of icariin in plasma after oral administration of Er-Xian decoction or pure icariin in rats