| Apoptosis induced by the inclusion complex of dihydromyricetin with hydroxypropyl-_-cyclodextrin in Human Hep G2 cells |
| 发布时间:2012-07-25 信息来源:admin 发布人:admin 点击次数:2932 |
|
Dihydromyricetin was a flavonoid with good prospect in food and medicine industry,but its poor water solubility limits its application. By forming the inclusion complex with hydroxypropyl-_-cyclodextrin, the solubility and stability of dihydromyricetin in water could be significantly improved. In this study, it was found that the complex could inhibit the proliferation of the human hepatocellular liver carcinoma Hep G2 cells and induce the significant change of cell cycle. After been treated by the complex, phosphatidylserine of Hep G2 could significantly translocate to the surface of the membrane. The increase of an early apoptotic population of Hep G2 cells was observed. It was concluded that the complex not only affected the progress of the cell cycle but also induced cells to enter into apoptosis. Key words: Dihydromyricetin, flavonoid, hydroxypropyl-_-cyclodextrin, complex, apoptosis. INTRODUCTION Flavonoids are one of the major active nutraceutical ingredients in plants and more than 4,000 kinds of flavonoids have been identified. However, only rutin and soy isoflavone can be widely used in food and medicine industry. Ampelopsis grossedentata, a particular wild plant in South China, is a kind of flavonoid-rich plant source; the main flavonoid in A. grossedentata leaves was identified as dihydromyricetin. The content of dihydromyricetin was more than 20% (Liu et al., 2009). In our previous study, with A. grossedentata leaves as raw material, a industrially significant method of preparing dihydromyricetin (DMY) was established. Now, A. grossedentata had been widely cultivated in China, and consequently, the large-scale production of dihydromyricetin and application in functional food and medicine will become possible. But the poor solubility and stability of dihydromyricetin in water restricted its application. Hydroxylpropyl-_-cyclodextrin (HP-_-CD), a hydroxyalkyl derivative of _-cyclodextrin, is an alternative to α-, β- andγ-cyclodextrin (CD), with improved water solubility (>500 g/L, 20°C). As the first CD derivatives approved by the FDA, HP-_-CDs have been widely applied in the food, agriculture and the pharmaceutical fields (Wen et al.,2010). It was found that the solubility and stability of dihydromyricetin in water could be significantly improved by forming the complex with hydroxypropyl-_-cyclodextrin (HP-_-CD). In order to determine the anticancer activity of the complex, the anti-proliferative effect of the complex and its apoptotic induction in human Hep G2 cells was investigated in this study. MATERIALS AND METHODS Materials and chemicals Dihydromyricetin (Purity > 95%) was gotten from our previous study.
A human hapatomacell line (Hep G2) was obtained from the Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences.Powdered Dulbecco modified eagle medium was purchased fromGIBCO (Grand Island, NY, USA). Foetal bovine serum (FBS) andantibiotics (penicillin and streptomycin mixture) were purchased fromHyclone Laboratories, Inc. Propidium iodide (PI) and 3-[4,5)-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide (MTT)were purchased from Sigma Chemical Co. An annexin V-FITCapoptosis detection kit was purchased from CLONTECH Company. Preparation of the inclusion complex of DMY with HP-_-CD DMY (1.6 g, 5 mM) and HP-_-CD (7 g, 5 mM) were dissolved in 50 mlof ethanol and stirred for 24 h. After ethanol was removed, theresidue was dissolved in 100 ml of water and filtered. The filtrate wasfrozen at -40°C for 24 h and then lyophilized (Alpha 1-4, Christ,Germany) and collected. The resultant light yellow power was collected as the inclusion complex of DMY with HP-_-CD. Cell culture and drug treatment Hep G2 cells were cultured in DMEM medium with 10% FBS, 100 UI/ml penicillin and 100 _g/ml streptomycin in humidified air at 37°C with 5% CO2. Exponentially growing Hep G2 cells were collected and re-suspended in fresh medium for 4 h and then exposed to various concentrations of the complex. MTT assay The cells were suspended at a final concentration of 2 × 105 cells/ml and seeded in 96-well microtiter plates. Various concentrations of compound were added to each well in triplicate. After incubation for the indicated times, MTT assay was employed to measure cell viability. After treatment, the cells were incubated with MTT (5 mg/ml) for 4 h. The formazan precipitate was dissolved in 100 _l DMSO and the absorbance at 490 nm was measured by a Benchmark microplate reader (Molecular Devices Corporation). The inhibition rate (IR %) was calculated as follows:
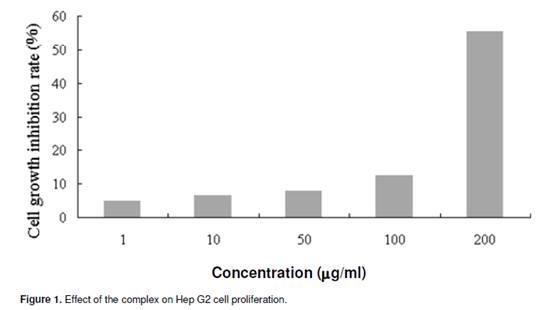
Flow cytometry analysis The flow cyctometry analysis was performed on a FACS Calibur Flow cytometer (BeckmanCoulter, USA). Cell pellets were fixed in 70% ethanol at -20°C for at least 12 h or overnight. After being washed twice with ice-cold PBS, they were incubated in RNase A/PBS (1 mg/ml) at 37°C for 30 min, and stained with PI (0.5 mg/ml) at room temperature for 15 min. The intracellular DNA was then labelled with PI and the PI fluorescence of individual nuclei determined by a FACSCalibur fluorescence-activated cell sorter at 488 nm excitation. Surface exposure of phosphatidylserine in apoptotic cells was measured by the annexin V-FITC apoptosis detection kit according to the manufacturer’s instructions. Additional exposure to PI made it possible to differentiate the early apoptotic cells from the late apoptotic cells. RESULT AND DISCUSSION Survival of cells was evaluated by using a system based on MTT, which was reduced by living cells to yield a soluble formazan product that could be detected colorimetrically. The inhibitory effects of the complex on the proliferation of Hep G2 cells were tested at different concentration for 48 h and the inhibition rate was determined (Figure 1). It induced a dose-dependent inhibitory effect. The inhibitory concentration 50% (IC50) was about 190 _g/ml.
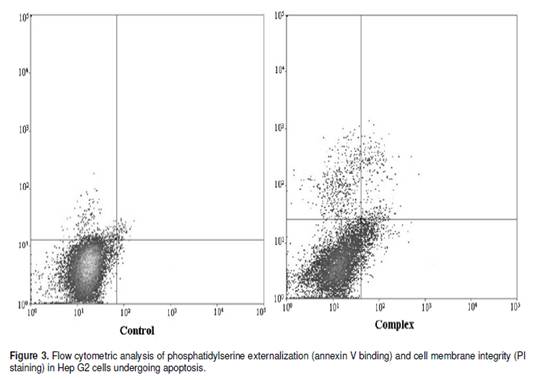
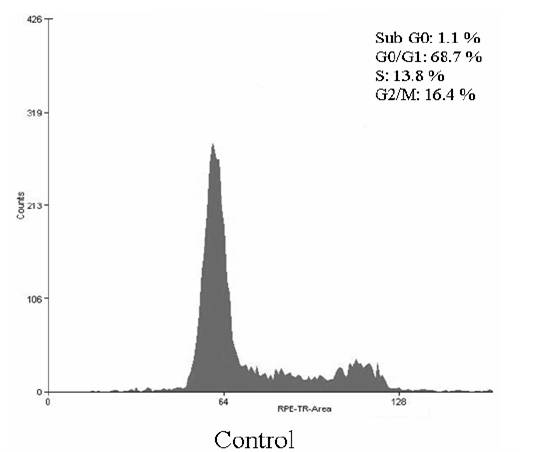
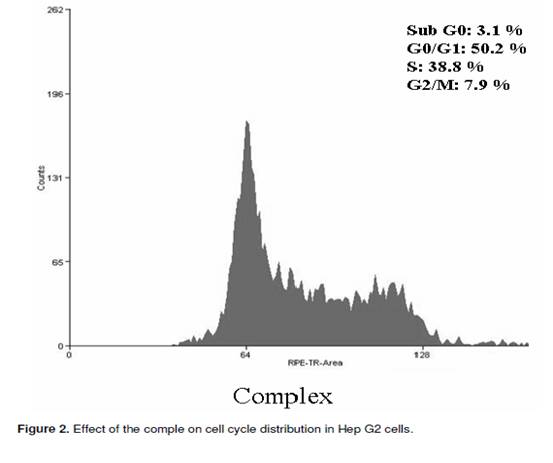
The cell cycle consists of four distinct phases: G1 phase, S phase (synthesis), G2 phase (collectively known as interphase) and M phase (mitosis). M phase is itself composed of two tightly coupled processes: mitosis, in which the cells chromosomes are divided between the two daughter cells, and cytokinesis, in which the cells cytoplasm divides in half forming distinct cells. Activation of each phase is dependent on the proper progression and completion of the previous one. To determine whether the Hep G2 cells treated with the complex undergo the apoptosis pathway, the cell distribution in the cell cycle was examined by PI staining. The significant increase of the population in the sub G0 and S phase in Hep G2 cells could be observed and the population of cells in G0/G1 and G2/M phases of the cell cycle decreased (Figure 2). Apoptosis is a highly regulated cell death process with characteristic biochemical features and membrane-bond apoptotic bodies. It occurs both during normal development and under certain pathological conditions in metazoans, and plays a crucial role in the maintenance of tissue homeostasis by the selective elimination of excessive cells. Evasion of apoptosis is an essential hallmark of cancer. Killing tumour cells through the induction of apoptosis has been recognized as a novel strategy for the identification of antitumour drugs and a valuable tool for cancer treatment (Liu et al., 2008). The hallmark of early apoptotic cells is the transverse redistribution of plasma membrane phosphatidylserine(PS). The annexin V binding assay was performed to detect the surface exposure of PS. In this study, the Hep G2 cells were treated with the complex (200 _g/ml) for 48 h. Figure 3 showed the FACS histogram with dual parameters including annexin V-FITC and PI. The dual parametric dot plots combining annexin V-FITC and PI fluorescence showed the viable cell population in the lower left quadrant (annexin V-negative/PI-negative), the early apoptotic cells in the lower right quadrant (annexin V-positive/PI-negative), and the late apoptotic cells in the upper right quadrant (annexin V-positive/ PI-positive). In the untreated Hep G2 cells, 0.7% of cells were the early apoptotic cells, 0.2% of cells were the late apoptotic cells. In the treated Hep G2 cells, the early and late apoptotic cells increased to 9.7% and 2.7%, respectively. The result suggested that the inclusion complex of dihydromyricetin with hydroxypropyl-_-cyclodextrin could induce cells into an apoptotic pathway resulting in the inhibition of proliferation of Hep G2. ACKNOWLEDGEMENTS The financial support provided by China Postdoctoral Science Foundation Funded Project (200902328), the National Natural Science Foundation of China (No.20806029) and the Fundamental Research Funds for the Central Universities, SCUT was greatly appreciated.
REFERENCES Liu B, Gao J, Zhao R, Ning Z, Wu Q (2009). Characterization and antioxidant activity of flavonoid-rich extracts from leaves of ampelopsis grossedentata. J. Food Biochem., 33(6): 808-820. Liu B, Du J, Zeng J, Chen C, Ning S (2009). Characterization and antioxidant activity of dihydromyricetin–lecithin complex. Eur Food. Res. Technol., 230(2): 325-331. Liu F, Liu S, Xu P, Xie Z, Cai G, Jiang Y (2008). Apoptosis induced by (di-isopropyloxyphoryl-Trp) 2-Lys-OCH3 in K562 and HeLa cells. J. Biosci., 33(1): 55-62. Liu B, Ma Y, Yuan C, Su C, Hu L, Wang J (2010). Characterization, stability and antioxidant activity of the inclusion complex of dihydromyricetin with hydroxypropyl-_-cyclodextrin. J. Food Biochem., (Accepted). Nagata S (1997). Apoptosis by death factor. Cell, 88(3): 355-365. Wen J, LIU B, Yuan E, Ma Y, Zhu Y (2010). Preparation and physicochemical properties of the complex of naringenin with hydroxypropyl-_-cyclodextrin. Molecules, 15(6): 4401-4407. Liu B, Zhao L, Wang A, Li G, Zhang P, Chen S (2009). Antioxidant and cytotoxic activity of dihydromyricetin from Ampelopsis Grossedentata leaves. Agro Food Ind Hi Tech., 20(3): 14-17 |







 Apoptosis induced by the inclusion complex of dihydromyricetin with hydroxypropyl-_-cyclodextrin in Human Hep G2 cells
Apoptosis induced by the inclusion complex of dihydromyricetin with hydroxypropyl-_-cyclodextrin in Human Hep G2 cells