| Variability of amygdalin content in seeds of sweet and bitter apricot cultivars in Turkey |
| 发布时间:2012-07-25 信息来源:admin 发布人:admin 点击次数:2831 |
In this study, amygdalin contents in the seeds of ten different bitter or sweet apricot cultivars were determined by high performance liquid chromatography (HPLC) for two years. The seeds of apricot cultivars were obtained from the Malatya Fruit Research Institute in Turkey. The results indicated that genetic variation was found among the cultivars. The amygdalin contents of bitter cultivars were found to be higher than those of sweet cultivars. As the average of two years, the amygdalin contents of the cultivars were determined as 6.354 g/100 g in Paviot (bitter), 5.914 g/100 g in Karacabey (bitter), 4.411 g/100 g in Alyanak (bitter), 1.584 g/100 g in Cologlu (sweet), 0.970 g/100 g in Cataloglu (sweet), 0.820 g/100 g in Aprikoz (sweet), 0.729 g/100 g in Sekerpare (sweet), 0.709 g/100 g in Kabaasi (sweet), 0.610 g/100 g in Ismailaga (sweet) and 0.604 g/100 g in Hacihaliloglu (sweet). Key words: Apricot (Prunus armeniaca L.), seed, amygdalin content. INTRODUCTION The origins of apricot (Prunus armeniaca L.) included in the genus Prunus in the subfamily Prunoideae in the family Rosaceae are the Central Asia, Western China, Iran and Caucasus. Apricot is a quite valuable fruit that can be processed into numerous different products (e.g. jam and fruit juice). Not only apricot is consumed in fresh and dried forms throughout the year, but also the seeds of its kernel are utilized in various ways. The apricot kernels that are sweet are consumed as dried fruits, whereas those that are bitter are used as raw materials in cosmetic and pharmaceutical industries (Asma and Misirli, 2007; Dwivedi and Ram, 2008). Cooking oil, benzaldehyde, activated carbon, amygdalin and hydrocyanic acid are obtained from the seeds of apricot (Asma and Misirli, 2007). Known as a cyanogenetic (cyanogenic) glycoside, amygdalin, D(-)-mandelonitrile β-gentiobioside, is found abundantly in many tissues, and especially seeds of species Prunus (e.g. cherry, peach, almond and apricot) (Haisman and Knight, 1967; Tatsuma et al., 2000). D-amygdalin is the main component of apricot seed extract (Kang et al., 2000). Overconsumption of seeds containing high amount of cyanogenetic glycoside might cause acute or chronic toxicity in human beings and animals (Silem et al., 2006). However, recently, interest in amygdalin is gradually increasing due to a derivative of amygdalin, that is, laetrile (vitamin B17), whose use as secondary cancer chemotherapy and antineoplastic agent has been encouraged (Suchard et al., 1998; Kang et al., 2000). Nevertheless, the cancer-preventive efficiency of laetrile has not been proved yet, and it is reported that no permission could be obtained from the U.S. Food and Drug Administration for the use of Vitamin B17 as a drug in the treatment of patients (Asma and Misirli, 2007). Apricot seeds contain various amounts of amygdalin depending on cultivars. It is reported that bitter cultivars contain higher amygdalin than sweet cultivars (Gomez et al., 1998). Femenia et al. (1995) reported that a high amount of amygdalin was found in bitter apricot cultivars, but not in seeds of sweet ones. Studies on this subject are rather limited. The aim of this study is to determine amygdalin contents in the seeds of bitter and sweet apricot cultivars. The obtained data might provide important information for the chemical characterization of these cultivars.
MATERIALS AND METHODS This study was conducted in 2005 and 2006. In the study, the seed samples of ten apricot culture cultivars were obtained from the trees in the apricot cultivar collection orchard located in the Malatya Fruit Research Institute in Turkey. Approximately 1 kg was sampled from each cultivar. The apricot cultivars in this study were previously identified considering the taste of their seeds (Asma, 2000). For this, the seeds of Alyanak, Karacabey and Paviot cultivars were reported to be bitter and the seeds of Aprikoz, Cataloglu, Cologlu, Hacihaliloglu, Ismailaga, Kabaasi and Sekerpare cultivars were reported to be sweet (Asma, 2000). Amygdalin analysis The seeds were hand-picked in the harvest time according to the maturation of cultivars. As soon as the fruits were sampled, their seeds were extracted and dried at room temperature for 2 days. In order to achieve standard drying, they were kept in an oven arranged at 30°C for 24 h. After the outer shell of the apricot kernel had been cracked out, the extracted seeds were ground by means of a blender. The ground samples were preserved at -80°C until they were analyzed. 2 g of apricot seed was extracted with 100 ml of methanol in the Soxhlet equipment for 6 h. Methanol was evaporated at 40°C in the evaporator. The residue was solved in 10 ml of mobile phase (CH3CN : H2O). The sample was passed through the Sep-Pak C18 cartridge preconditioned with CH3CN and H2O. The solution was diluted 500 times, and 10 μl of sample was injected into the high performance liquid chromatography (HPLC) (Gomez et al., 1998). Conditions for the HPLC were: Detector: SPD-10AVvp diode array detector (λmax = 238 nm), system controller: SCL-10 Avp, Pump: LC-6AD, Column: ACE 5-C18 (250 x 4.6 mm) 5 μ, mobile phase: 75% ACN, flow rate: 0.9 ml/min, injection volume: 20 μl, column temperature: 25°C. Amygdalin (Sigma) was used as a standard. The amygdalin content was determined by comparing the obtained peaks with the standards according to their relative rise and time. The experiment was conducted with three replications. The results were analyzed in the Minitab software (MINITAB Inc.) program according to the method of analysis of variance, and the resulting significant differences were checked according to the F test. Intergroup difference was determined in accordance with the Tukey’s test and indicated by means of letters. Yildirim and Askin 6523 RESULTS AND DISCUSSION The results of the analysis of variance are presented in Table 1 and the means of the amygdalin contents of the studied apricot cultivars are presented in Table 2. The results of the analysis of variance indicated that the cultivars and the “cultivar x year” interaction were found to be statistically significant at p < 0.01 significance level with regards to amygdalin content, while the years were found to be statistically significant at p < 0.05 significance level (Table 1). In this study, there were significant differences in the amygdalin contents of sweet and bitter apricot cultivars for both years (Table 2). The Tukey’s test showed that seeds of the bitter apricot cultivars (average 5.559 g/100g) contained significantly more amygdalin than seeds of the sweet cultivars (average 0.861 g/100g). These results supported previous studies reporting that bitter apricot seeds contained a high level of amygdalin (Gomez et al., 1998; Feminia et al., 1995). Also, these results were higher than the results that Ohtsubo and Ikeda (1994) reported for same bitter almonds (47 mg/g). On the other hand, a small amount of amygdalin was found in seeds of the sweet cultivars (Table 2). These results showed similarity with the findings of Gomez et al. (1998) who reported that seeds of sweet cultivars contained a low level of amygdalin. Conversely, Feminia et al. (1995) reported that sweet apricot seeds did not contain any amygdalin. Also, Yildirim et al. (2010) found that the amygdalin contents of seeds of sweet almond genotypes ranged from 1.53 to 11.25 mg/g and Dicenta et al. (2002) reported that bitter almond geno-types had higher amygdalin content than seeds of sweet almond genotypes. This clarifies the relatively slight bitterness of the seeds that were described as sweet. Moreover, Gomez et al. (1998) reported that there might be factors other than amygdalin that can mask the bitter taste in completely sweet seeds. When the amygdalin contents based on bitter cultivars were evaluated, no significant differences were observed between the two years (Table 2). Also, there was genetic difference among bitter cultivars. The highest amygdalin content was determined in Paviot (avarage 6.353 g/100 g), followed by Karacabey (average 5.914 g/100 g) and Alyanak (average 4.411 g/100 g). Although Paviot and Karacabey cultivars were placed in the same statistical group based on the Tukey’s test, Alyanak was placed differently in a statistical group than the Paviot and Karacabey cultivars. When the amygdalin contents based on sweet cultivars were examined, significant difference was found among the sweet cultivars in 2005, while this difference remained insignificant in 2006. As a matter of fact, higher amygdalin content was found in Cologlu cultivar (2.181 g/100 g) than in other sweet cultivars in 2005. However, the lowest amygdalin content, in the year concerned, was determined REFERENCES Asma BM, Misirli A (2007). Kayısı cekirdegi. Hasad Bitkisel Üretim. 22(261): 55-58. Asma BM (2000). Apricot growing. Malatya, Turkey, Evin Publishers (in Turkish). Dicenta F, Martinez-Gomez P, Grane N, Martin ML, Leon A, Canovas JA, Berenguer V (2002). Relationship between cyanogenic compound in kernels, leaves and roods of sweet and bitter kernelled almonds. J. Agric. Food Chem. 50(7): 2149-2152. Dwivedi DH, Ram RB (2008). Chemical Composition of Bitter Apricot Kernels from Ladakh, INDIA. Acta Hort. (ISHS) 765: 335-338, Femenia A, Rossello C, Mulet A, Canellas J (1995). Chemical Composition of Bitter and Sweet Apricot Kernels. J. Agric. Food Chem. 43(2): 356-361. Haisman DR, Knight DJ (1967). The Enzymic Hydrolysis of Amygdalin. Biochem. J. 103(2): 528-534. Ohtsubo T, Ikeda F (1994). Seasonal changes of cyanogenic glikosides in mume seeds. J. Jpn. Soc. Hortic. Sci. 62: 695-700. Siami S, Lapsley K, Jeong WS, LaChance PA, Ho CT, Rosen RT (2002). Antioxidative phenolic compounds isolated from almond skins (Prunus amygdalus Batsch). J. Agric. Food Chem. 50: 2459-2463. Silem A, Günter HO, Einfeldt J, Boualia A (2006). The occurrence of mass transport processes during the leaching of amygdalin from bitter apricot kernels: detoxification and flavour improvement. Int. J. Food Sci. Technol. 41: 201-213. Suchard JR, Wallace KL, Gerkin RD (1998). Acute Cyanide Toxicity Caused by Apricot Kernel Ingestion. Ann. Emergency Med, 32(6): 742-744. Tatsuma T, Komori K, Yeoh HH, Oyama N (2000). Disposable test plates with tyrosinase and b-glucosidases for cyanide and cyanogenic glycosides. Analytica Chimica Acta, 408: 233-240 Yildirim AN, San B, Koyuncu F, Yildirim F (2010). Variability of phenolics, α-tocopherol and amygdalin contents of selected almond (Prunus amygdalus Batsch.) genotypes. J. Food Agric. Environ. 8(1): 76-79. |







 Variability of amygdalin content in seeds of sweet and bitter apricot cultivars in Turkey
Variability of amygdalin content in seeds of sweet and bitter apricot cultivars in Turkey 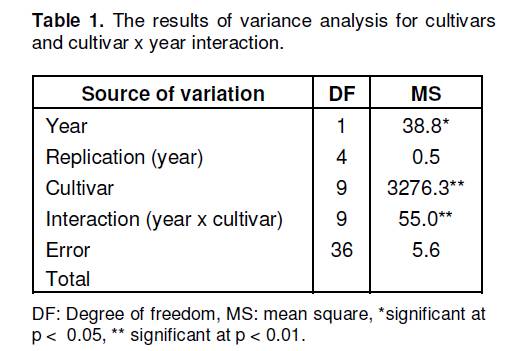
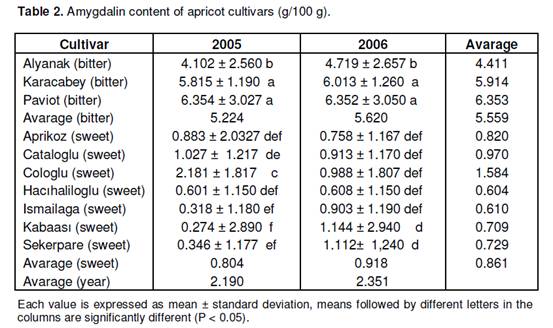 in Kabaasi cultivar (0.274 g/100 g). In 2006, the amygdalin contents of sweet cultivars ranged from 1.144 g/100 g (Kabaasi) to 0.608 g/100 g (Hacıhaliloglu). In this study, while amygdalin was found at similar levels in Aprikoz, Cataloglu and Hacihaliloglu cultivars during both years, a lower level of amygdalin was found in Ismailaga, Kabaasi and Sekerpare cultivars in 2005 than in 2006. These differences in amygdalin content are considered to be due to the differences in the harvest time when the fruit was collected. As a matter of fact, Frehner et al. (1990) reported that the cyanogenic content of seeds might vary significantly throughout maturation and that an increase occurred with maturation in almond seeds (Gomez et al., 1998). In conclusion, genetic variation was observed among the cultivars. It was shown that seeds of bitter cultivars contained quite a high level of amygdalin than seeds of sweet cultivars. In this investigation, seeds of bitter apricot cultivars, Paviot and Karacabey, contained the highest amygdalin content among all apricot cultivars. Seeds of sweet cultivars, Hacıhaliloglu and Ismailaga, contained the lowest amygdalin. Also, sweet cultivar Cologlu contained higher amygdalin than other sweet cultivars.
in Kabaasi cultivar (0.274 g/100 g). In 2006, the amygdalin contents of sweet cultivars ranged from 1.144 g/100 g (Kabaasi) to 0.608 g/100 g (Hacıhaliloglu). In this study, while amygdalin was found at similar levels in Aprikoz, Cataloglu and Hacihaliloglu cultivars during both years, a lower level of amygdalin was found in Ismailaga, Kabaasi and Sekerpare cultivars in 2005 than in 2006. These differences in amygdalin content are considered to be due to the differences in the harvest time when the fruit was collected. As a matter of fact, Frehner et al. (1990) reported that the cyanogenic content of seeds might vary significantly throughout maturation and that an increase occurred with maturation in almond seeds (Gomez et al., 1998). In conclusion, genetic variation was observed among the cultivars. It was shown that seeds of bitter cultivars contained quite a high level of amygdalin than seeds of sweet cultivars. In this investigation, seeds of bitter apricot cultivars, Paviot and Karacabey, contained the highest amygdalin content among all apricot cultivars. Seeds of sweet cultivars, Hacıhaliloglu and Ismailaga, contained the lowest amygdalin. Also, sweet cultivar Cologlu contained higher amygdalin than other sweet cultivars.