| Isolation and Quantitation of Amygdalin in Apricot-kernel and Prunus Tomentosa Thunb. by HPLC with Solid-Phase |
| 发布时间:2012-07-24 信息来源:admin 发布人:admin 点击次数:5544 |
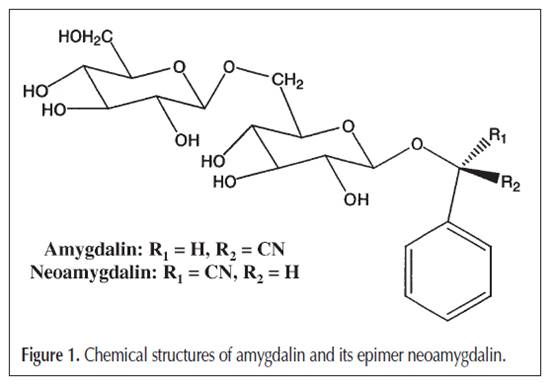
Abstract Apricot-kernel and Prunus Tomentosa Thunb. are traditional Chinese herb medicines that contain amygdalin as their major effective ingredient. In this report, three methods for the extraction of amygdalin from the medicinal materials are compared: ultrasonic extraction by methanol, Soxhlet extraction by methanol, and reflux extraction by water. The results show that reflux extraction by water containing 0.1% citric acid is the best option. The optimal reflux time is 2.5 h and water bath temperature is 60°C. The solid-phase extraction method using C18 and multiwalled carbon nanotube as adsorbents is established for the pretreatment of reflux extract, and the result shows that the two adsorbents have greater adsorptive capacity for amygdalin and good separation effect. In order to quantitate amygdalin in Apricot-kernel and Prunus Tomentosa Thunb., a reversed-phase high-performance liquid chromatography method using methanol–water (15:85, for 30 min and pure methanol after 30 min) as mobile phase is developed and a good result is obtained. Introduction Apricot-kernel is the dry and mature seed of Prunus armeniaca L. and Prunus armeniaca L. var. Ansu Maxim. It has been prescribed in many traditional Chinese medicines for its antitussive, expectorant, and laxative functions (1). Prunus Tomentosa Thunb. is the dry and mature seed of Prumus humilis Bge., Prunus japonica Thunb., and Prunus pedunculata Maxim. It has diuresis and moistening dryness activities and is used in treating oedema and dermatophytosis (2). The major effective ingredient of these two herb medicines is amygdalin (D-mandelonitrile-b-Dgentiobioside) (as shown in Figure 1), which has antitussive and lubricant activities. It is decomposed by the action of b-D-glucosidase to yield hydrocyanic acid, which reflexively stimulates the respiratory center and produces antitussive and antiasthmatic effects (3). Recently, it has been reported that amygdalin can kill cancer cells selectively at the tumor site without systemic toxicity (4). Amygdalin can be dissolved in water and methanol easily. Traditionally, the extraction method of the chemical ingredients in the medicinal materials is decoction in boiling water. But the common techniques for extracting amygdalin from Apricotkernel and Prunus Tomentosa Thunb. for the analysis of amygdalin are ultrasonic extraction or Soxhlet extraction by methanol (5,6). This is because some amygdalin is decomposed into benzaldehyde, HCN, and glucose by emulsion (a hydrolysis enzyme both in Apricot-kernel and Prunus Tomentosa Thunb.), and some are converted into its epimers, neoamygdalin (L-mandelonitrile- b-D-gentiobioside) (Figure 1) during the process of decoction in water. Recently, Hwang et al. established an optimum condition for inhibiting the conversion of amygdalin to neoamygdalin in Tonin (Persicae Semen) by changing the pH. They added 0.1% citric acid in the boiling water and prevented the loss of content of amygdalin (7). They also studied the optimum extraction condition of amygdalin without enzymatic hydrolysis from Tonin. They concluded that the extraction yield of amygdalin was highest when using the size larger than half (8). The determination of amygdalin was performed mainly by
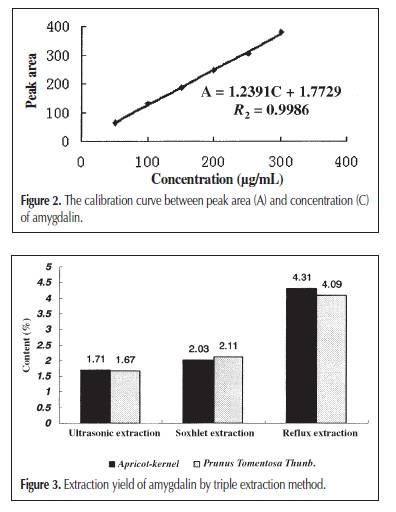
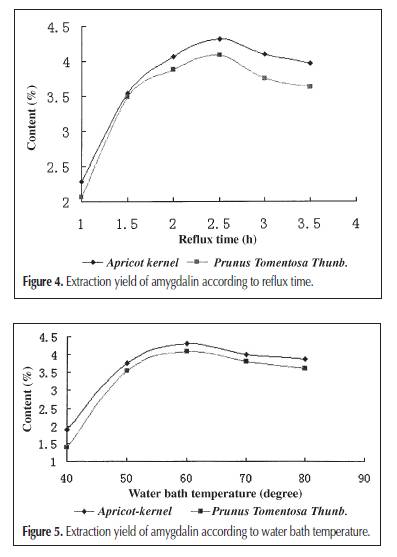
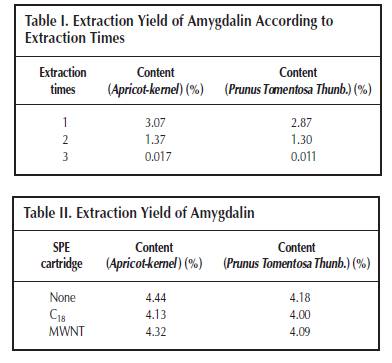
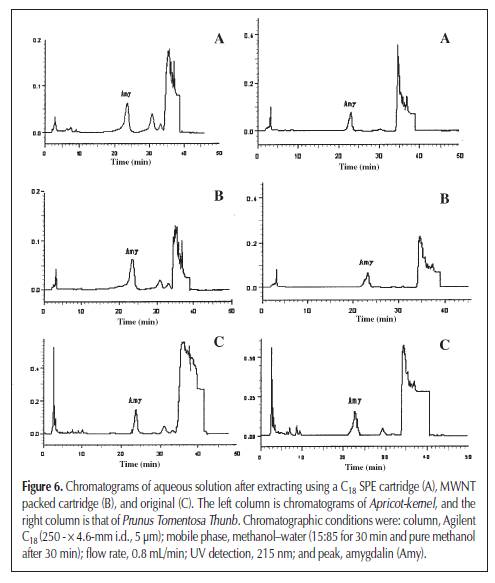
high-performance liquid chromatography (HPLC) (9). Although other analytical methods are reported, such as gas chromatography (10) and capillary electrophoresis (3). HPLC is the most convenient method, usually with a C18 column as the stationary phase and a mixture of methanol (acetonitrile) and water as the mobile phase. Solid-phase extraction (SPE) is a universal sample pretreatment method. It’s advantages include solvent retention, high selectivity, and easy operation. It was widely applied in the separation and concentration of target compounds in complex samples, such as entironmental water and herb medicine (11,12). The aim of this study was to establish an optimal method for the extraction of amygdalin from Apricot-kernel and Prunus Tomentosa Thunb.; a sample pretreatment method using SPE, C18, and multiwalled carbon nanotube (MWNT) as adsorbents; and a separation and analysis method using HPLC. This is the first report to separate amygdalin from herb medicines using SPE with MWNT as the adsorbent. Experimental Chemicals and reagents Apricot-kernel and Prunus Tomentosa Thunb. were purchased from Tong Ren Pharmacy (Beijing, China). Amygdalin was purchased from Beijing Chemical Reagent Company (Beijing, China). MWNT was obtained from Reaction Engineering Lab of Chemical Engineering Department, Tsinghua University (Tsinghua, China). HPLC-grade methanol was used, and the other reagents and solvents were of guaranteed or analytical grade. Apparatus and conditions HPLC An HP 1100 chromatographic system consisting of a quaternary pump, degasser, diode array detector, and HP ChemStation Data system (Hewlett-Packard, Palo Alto, CA) was used. Separation was achieved on a Agilent C18 column (250 × 4.6-mm i.d., 0.45 μm). The mobile phase consisted of methanol–water (15:85 for 30 min and pure methanol after 30 min) and the flow rate was 0.8 mL/min. The mobile phase was filtered before use by a Millipore vacuum filter system equipped with a 0.45-μm filter (Billerica, MA). Because the maximal absorption of amygdalin was at 215 nm, the detection wavelength was set at 215 nm. The column temperature was 30°C. The injected volume of samples was 20 μL, by loop. SPE The SPE instrument was a RapidTrace SPE workstation (Zymark Company, Hopkinton, MA). Calibration curve and detection limit Amygdalin was dissolved in methanol and six different standard solutions containing 50, 100, 150, 200, 250, and 300 μg/mL of it were obtained. The calibration curve was constructed according to the peak area and the concentration of amygdalin. Then the standard solution of the lowest concentration was diluted gradually and injected into the instrument to determine the detection limit when the signal-to-noise ratio is 3. Extraction method Ultrasonic extraction Apricot-kernel and Prunus Tomentosa Thunb were prepared in the form of small pieces. Each sample (1 g) was immersed in 10 ml methanol and ultrasonicated for 30 min. Extraction was repeated two times. The combined methanol extract was obtained after filtration and made volume up to 25 mL. Soxhlet extraction Small pieces of Apricot-kernel and Prunus Tomentosa Thunb. (3 g of each) were Soxhlet extracted by 100 mL methanol for 5 h, in a water bath at 70°C. The methanol extract was obtained after filtration and diluted to 100 mL. Reflux extraction Small pieces of Apricot-kernel and Prunus Tomentosa Thunb. (3 g of each) were reflux extracted by 100 mL water with 0.1% citric acid for 2.5 h in the condition of a 60°C water bath. The water extract was collected after filtration. The residue was dissolved in 100 mL water with 0.1% citric acid and the previously mentioned process was repeated. The two times water extract was then combined and diluted to 200 mL. SPE method Prior to a preconcentration step, the C18 and MWNT packed cartridge was washed with 5 mL of methanol at a flow rate of 4 mL/min and activated with 5 mL of water at a flow rate of 4 mL/min. Then, 5 mL sample solution was passed through the preconditioned cartridge at a flow rate of 4 mL/min. After the sample solution had passed through, the cartridge was washed with 5 mL of 10% methanol aqueous solution at a flow rate of 4 mL/min to remove coadsorbed matrix materials from the cartridge. The analytes retained on the SPE packing materials were then eluted with 5 mL of methanol at a flow rate of 4 mL/min, and the eluate was diluted to 5 mL. Finally, 20 μL of methanol eluate was injected into the HPLC system for determination. Results and Discussion Separation of amygdalin by reversed-phase HPLC It was necessary to establish a suitable HPLC method to determine amygdalin in the herb medicines. An ODS column (C18, Agilent) and methanol–water as mobile phase were used. It was found that when the ratio of methanol and water was 15:85, amygdalin could be completely separated with the other ingredients. But if the ratio of methanol was a little large, amygdalin would not be separated well. If the ratio of methanol was small, it would lead to long analytical time. Because amygdalin was the only analyte, other ingredients had to be eluted as quickly as possible to cut down on analytical time when the peak of amygdalin was finished. For this reason, a gradient elution method (methanol–water is 15:85 for 30 min and pure methanol after 30 min) was also attempted, and a good chromatographic separation was obtained within 50 min (Figure 2). The calibration curve between peak area (A) and concentration (C) of amygdalin showed excellent linearity (r2 = 0.9986), and the detection limit was 0.2 μg/mL (Figure 3). Comparing three solvent extraction methods for amygdalin Three extraction methods—including ultrasonic extraction by methanol, Soxhlet extraction by methanol, and reflux extraction by water—were compared for amygdalin. Figure 4 shows the extraction yield, presented as the mass ratio of amygdalin to medicinal materials. Among the extraction yield of amygdalin, reflux extraction by water was the maximum, and ultrasonic extraction by methanol was the minimum. Water is a good solution for extracting ingredients of crude herb medicine, because it is known that it can extract almost all of the ingredients. Though methanol is also a common solution for extraction, and amygdalin can be easily dissolved in it too. However, if all the factors are contemplated (such as economy, accurate quantitation, and traditional method of decoction in boiling water), reflux extraction by water is the best choice for the Apricot-kernel and Prunus Tomentosa Thunb. Optimum conditions on the reflux extraction of amygdalin by water The main factors that influenced the amygdalin extraction yield by refluxing on a water bath are reflux time, water bath temperature, and extraction times. Citric acid (0.1%) was added in the aqueous solution to prevent amygdalin from decomposition and inhibit the conversion of amygdalin to neoamygdalin (7). Several different reflux times and water bath temperatures were selected. The extraction yield of amygdalin, according to reflux It could be observed that a 2.5-h reflux time and 60°C water bath temperature are the most suitable reflux extraction conditions. A short reflux time and low water bath temperature would decrease the extraction yield of amygdalin. A long reflux time and high water bath temperature would cause some amygdalin to convert into its epimers or decompose in the aqueous solution. Extraction times were also investigated. Table I lists the extraction yield of amygdalin according to extracting times. It can be concluded that amygdalin was almost completely extracted after two extraction processes. SPE The solution extracted by water pollutes the separation column so as to reduce the column effect and also make it more difficult to separate the ingredients of the herbal medicine, because the components in it are too complex. Therefore, an SPE was used to attempt to solve this problem. C18 is one of the most common adsorbents used as the solid phase of SPE. It is applied to many compounds from nonto medium polarity. The principle of extraction is similar to that of LC. Figure 2A is the chromatogram of an aqueous solution after extraction using a C18 SPE cartridge. It could be found that the chromatographic peaks of other ingredients, especially those that elute prior to amygdalin, are weakened greatly, compared with those of the original (Figure 2C), and the content of amygdalin is almost the same. The result indicated that the C18
SPE cartridge is a good adsorbent for amygdalin and it could be applied in the pretreatment method for the separation of amygdalin from herb medicine. MWNT is a new material. Recently, Cai et al. used MWNTas SPE adsorbent firstly for determination of bisphenol-a,4-n-nonylphenol, and 4-tert-octylphenol (13). The compound
that has a delocalized ¹-bond structure, such as benzene ring, could easily combine with MWNT and achieve good adsorptive effect, for the ¹ bond at the surface of the benzene ring has an interaction with the ¹ bond at the surface of the MWNT. MWNT was used as the adsorbent in the pretreatment method for the separation of amygdalin from other ingredients in the Apricotkernel and Prunus Tomentosa Thunb., because amygdalin has a benzene ring structure (Figure 1), according to the absorptive capability of MWNT (that it is strong to the compounds that have a benzene ring while weak to others). Figure 2B is the chromatogram of aqueous solution after extracting using MWNT packed cartridge. The result indicated that MWNT is a better adsorbent because amygdalin can be adsorbed in it more strongly and than other ingredients, compared with C18, and MWNT could be used as the solid phase for SPE to obtain better HPLC analysis result for isolation and quantitation of amygdalin in Apricotkernel and Prunus Tomentosa Thunb. Table II lists the extraction yield of amygdalin in Apricot-kernel and Prunus Tomentosa Thunb. Conclusion An HPLC method for quantitative analysis of amygdalin in Apricot-kernel and Prunus Tomentosa Thunb. is developed. An SPE method that uses C18 and MWNT as adsorbents after refluxing by water was established in order to obtain a better separation effect and to protect the chromatographic column. The results showed that these two adsorbents were effective, especially MWNT. This method could be used to determine amygdalin in Apricot-kernel and Prunus Tomentosa Thunb. because it is convenient and rapid. References 1. Pharmacopoeia Committee of P.R. China. Pharmacopoeia of P.R. China Part I, 2000 edition. Chemical Industry Press, Beijing, China, 2000, pp. 160–61. 2. Pharmacopoeia Committee of P.R. China. Pharmacopoeia of P.R. China Part I, 2000 edition. Chemical Industry Press, Beijing, China, 2000, pp.166–67. 3. T. Isozaki, Y. Matano, K. Yamamoto, N. Kosaka, and T. Tani. Quantitative determination of amygdalin epimers by cyclodextrin-modified micellar electrokinetic chromatography. J. Chromatogr. A 923: 249–54 (2001). 4. K.N. Syrigos, B.G. Rowlinson, and A.A. Epenetos. In vitro cytotoxicity following specific activation of amygdalin by beta-glucosidase conjugated to a bladder cancer-associated monoclonal antibody. Int. J. Cancer 78: 712–19 (1998). 5. P. Zhen, D.B. Wang, T.J. Jia, and X.M. Bai. Analysis on amygdalin of Prunus Tomentosa Thunb. By HPLC. J. Zhangjiakou TCM. Coll. 19: 13–14 (2002). 6. S. Ma and C.H. Li. Determination of amygdalin in Apricot-kernel. Chin. J. Exp. Trad. Med. Form. 6: 16–17 (2000). 7. E.Y. Hwang, J.H. Lee, Y.M. Lee, and S.P. Hong. Reverse-phase HPLC separation of D-amygdalin and neoamygdalin and optimum conditions for inhibition of racemization of amygdalin. Chem. Pharm. Bull. 50: 1373–75 (2002). 8. E.Y. Hwang, S.S. Lee, J.H. Lee, and S.P. Hong. Development of quantitative extraction method of amygdalin without enzymatic hydrolysis from Tonin(Persicae Semen) by high performance liquid chromatography. Arch. Pharm. Res. 25: 453–56 (2002). 9. K. Wasserkrug and Z. ElRassi. High-performance liquid phase separation of glycosides. 1. Reversed phase chromatography of cyanogenic glycosides with uv and pulsed amperometric detection. J. Liq. Chromatogr. Related Technol. 20: 335–49 (1997). 10. T. Cirns, J. Froberg, and S. Gonzales. Analytical chemistry of amygdalin. Anal. Chem. 50: 317–22 (1978). 11. P. Louchouarn, S. Opsahl, and R. Benner. Isolation and quantification of dissolved lignin from natural waters using solid-phase extraction and GC/MS. Anal. Chem. 72: 2780–87 (2000). 12. J.P. Lai, X.W. He, Y. Jiang, and F. Chen. Preparative separation and determination of matrine from the Chinese medicinal plant Sophora flavescens Ait by molecularly imprinted solid-phase extraction. Anal. Bioanal. Chem. 375: 264–69 (2003). 13. Y.Q. Cai, G.B. Jiang, J.F. Liu, and Q.X. Zhou. Multiwalled carbon nanotubes as a solid-phase extraction adsorbent for the determination of bisphenol a, 4-n-nonylphenol, and 4-tert-octylphenol. Anal. Chem. 75: 2517–21 (2003). |







 Isolation and Quantitation of Amygdalin in Apricot-kernel and Prunus Tomentosa Thunb. by HPLC with Solid-Phase
Isolation and Quantitation of Amygdalin in Apricot-kernel and Prunus Tomentosa Thunb. by HPLC with Solid-Phase