| The antiproliferative activity of aloe-emodin is through p53-dependent and p21-dependent apoptotic pathway in human hepatoma cell lines |
| 发布时间:2012-07-23 信息来源:admin 发布人:admin 点击次数:4678 |
Abstract The aim of this study is to investigate the anticancer effect of aloe-emodin in two human liver cancer cell lines,Hep G2 and Hep 3B. We observed that aloe-emodin inhibited cell proliferation and induced apoptosis in both examined cell lines, but with different the antiproliferative mechanisms. In Hep G2 cells, aloe-emodin induced p53 expression and was accompanied by induction of p21 expression that was associated with a cell cycle arrest in G1 phase. In addition, aloe-emodin had a marked increase in Fas/APO1 receptor and Bax expression. In contrast, with p53-deficient Hep 3B cells, the inhibition of cell proliferation of aloe-emodin was mediated through a p21-dependent manner that did not cause cell cycle arrest or increase the level of Fas/APO1 receptor, but rather promoted aloe-emodin induced apoptosis by enhancing expression of Bax. These findings suggest that aloe-emodin may be useful in liver cancer prevention.D 2002 Elsevier Science Inc. All rights reserved. Keywords: Aloe-emodin; p53; p21/WAF1; Fas/APO1; Apoptosis; Hepatoma cell line Introduction Hepatocellular carcinoma (HCC) is one of the most lethal malignancies, and is also one of the four most prevalent malignant diseases of adults in China, Taiwan, Korea, and Sub-Africa [1,2]. Several etiologic factors have been classified as high-risk factor in association with HCC, including exposure to aflatoxin B1, and infection with hepatitis B virus and hepatitis C virus [3,4]. Aloe-emodin (1,8dihydroxy-3-hydroxymethyl-9,10-anthracenedione) is an anthraquinone compound that is present in some traditional medicinal plants such as Rhei Rhizoma. Many studies have indicated that aloe-emodin has laxative, antifungal, anitibacterial, antiviral, and hepatoprotective effects [5–9]. Recent study has indicated that aloe-emodin has a specific in vitro and in vivo antineuroectodermal tumor activity. The anticancer mechanism consists of the induction of apoptosis, and the selectivity against neuroectodermal tumor cells is found on a specific energy-dependent pathway of drug incorporation [10]. However, the underlying mechanism of action of aloe-emodin has remained largely unknown. Fas/APO1, a type I membrane protein in the tumor necrosis factor (TNF) receptor family of cell surface protein, is the major cell surface molecular protein that mediates external ligand-stimulated apoptosis [11,12], and is ubiquitously expressed in a variety of tissues, including the liver, thymus, heart, lungs, and ovaries [13,14]. Many anticancer agents induce apoptosis in hepatoma cells through the Fas-Fas ligand system [15]. In addition, tumor suppressor gene p53 is another important molecule in the process of apoptosis. In fact, a number of studies have raised the possibility that cells lacking p53 activity might be more readily resistant to cancer chemotherapy [16–18]. Once p53 is activated, the outcome of the cellular response is either cell cycle arrest or apoptosis. In some type of cells, p53 increases the amount of p21/WAF1 protein. p21 is a cell cycle regulator that contributes to the arrest of cell in G1 phase by inhibition of cyclin-cdk complex. In certain cells, the activation of p53 leads to apoptosis. It is mediated by transcriptional transactivation of p53 for many apoptotic genes, including Fas/APO1, Bax, IGF-BP3 and PIG3. Moreover, the level of antiapoptotic factor Bcl-2, is decreased by p53. Therefore, these actions of p53 commit cell to apoptosis. [19]. To further elucidate the antiproliferative activity of aloe-emodin, we examined its effect on cell proliferation, cell cycle kinetics, and apoptosis in two well-characterized human hepatoma cell lines, Hep G2 and Hep 3B. In addition, to establish the anticancer effects of aloe-emodin, we also assayed several molecules, including p53, p21, Bcl-2, Bax, and Fas/APO1, that are strongly associated with the pathway of apoptosis and affect the chemosensitivity of tumor cells to anticancer agents. Our results show that the anticancer activity of aloe-emodin in Hep G2 was through a p53-triggerd apoptotic manner, and in Hep 3B, it was mediated by p21-dependent apoptotic pathway. Methods Test compound Aloe-emodin was obtained from Sigma Chemical (USA), dissolved in dimethyl sulfoxide (DMSO) and stored at 20 jC. Final concentrations of aloe-emodin used for different experiments were prepared by diluting the stock with Dulbecco’s modified Eagle’s medium (DMEM) (Sigma Chemical). Control cultures received the carrier solvent (0.1% DMSO). Cell lines and culture Hep G2 (American Type Culture Collection [ATCC] HB8065) and Hep 3B (ATCC HB 80640) were maintained in DMEM supplemented with 10% FCS (GIBCO BRL), 100 units/ml penicillin G, 100 Ag/ml streptomycin, and 0.25 Ag/ml amphotericin B (GIBCO BRL), in a 37 jC humidified incubator under an atmosphere of 5% CO2 in air.P.-L. Kuo et al. / Life Sciences 71 (2002) 1879–1892 1880Assay for cell proliferation inhibition Cell proliferation inhibition was assessed by XTT (sodium 3V-[1-(phenylamino-carbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro)benzene-sulfonic acid hydrate) (Roche Molecular Biochemicals, Germany) assay. Briefly, cell lines were subcultured into a 96-well plate with 1 [1] 104 cells per well in 100 Al medium. After 24 h of incubation, the medium in the 96-well plate was discarded, replaced by 90 Al of new medium, and 10 Al of aloe-emodin was then added to each well to final concentrations of 1,5, 10, and 20 Ag/ml each in triplicate. After the addition of the sample, the plates were incubated in a 37 jC humidified incubator under an atmosphere of 5% CO2 for 12, 24, 48, and 72 h. At the end of the incubation, the medium containing the various concentrations of aloe-emodin was discarded, and the cells were washed with PBS. 50 Al of XTT test solution prepared by mixing 5 ml of XTT-labeling reagent and 100 Al of electron coupling reagent was then added to each well. After 6 h of incubation in a 37 jC and 5% CO2 incubator, the absorbance was measured on an ELISA reader (Multiskan EX, Labsystems) at a test wavelength of 492 nm, with a reference wavelength of 690 nm [20,21]. Assay for cell cycle distribution Hep G2 and Hep 3B cells were cultured in triplicate in 6-well plates at a concentration of 5 [1] 105 /well. After 24 and 48 h, cells were treated with 10, or 20 Ag/ml of aloe-emodin, in parallel with cells grown in the absence of aloe-emodin to determine effects on cell cycle distribution. Cells were collected and fixed with 70% ethanol. Cell pellets were suspended in 2 AAlof10 Ag/ml RNase (Sigma Chemical) containing 0.5% Triton (J.T. Baker) plus the same volume of 20 Ag/ml propidium iodide (Sigma Chemical) and then incubated in the dark at room temperature for 30 min. Cell suspensions were filtered through a 60-Ammesh filter (Spectrum Medical, CA). Data acquisition and analysis were performed on an EPICS flow cytometer (Coulter Electronics). Data from 10,000 cells were collected for each data file. Cell cycle analysis was performed with Multicycle software (Phoenix Flow Systems, San Diego, CA) [22]. Assay for apoptosis by nucleosome ELISA For the quantification of apoptotic cells, we used the Nucleosome ELISA kit from Oncogene (Calbiochem, Cambridge, MA, USA), essentially in accord with to the manufacturer’s protocol. Briefly, Hep G2 and Hep 3B cells were treated with 10 or 20 Ag/ml aloe-emodin for 6, 12, 24, and 48 h, then samples of cell lysate and the biotinylated detector antibody were placed (triplicated) in the 96-well plate coated DNA-binding protein with final 1 [1] 106 cells per well. During this assay, mono- and oligonucleosomes were captured on pre-coated DNA-binding proteins. Anti-histones 3 (H3) biotin-labeled antibodies were then bound to the histone component of captured nucleosomes, and were detected following incubation with streptavidin-linked horseradish peroxidase (SA-HRP) conjugate. HRP catalyzed the conversion of colorless tetramethylbenzidine to blue. The addition of Stop Solution changed the color to yellow, the intensity of which was proportional to the number of nucleosomes in the sample. Absorbance was measured on an ELISA reader at a test wavelength of 450 nm. By comparing the absorbance obtained from a sample containing an unknown amount of nucleosomes with that obtained from the standards, one can assign a nucleosome unit value to the unknown sample. In addition, one can assign an apoptotic index (ratio) to an unknown sample when the absorbance of the treated (apoptotic) sample was divided by that of the untreated (control) sample [23]. P.-L. Kuo et al. / Life Sciences 71 (2002) 1879–1892 1881 ELISA assay of p53, p21, and Fas protein For the detection of p53, p21, and Fas protein, we used the p53 pan ELISA kit (Roche Molecular Biochemicals, Germany), WAF1 ELISA and Fas/APO-1 ELISA kits (Calbiochem, Cambridge, MA, USA), respectively. Briefly, cells were treated with 10 or 20 Ag/ml of aloe-emodin for 6, 12, 24, and 48 h, the samples to be assayed (the lysate from approximately 4 [1] 105 cells from each culture plate) and the biotinylated specific detector antibody (mouse monoclonal) were placed (triplicated) in 96 well microtiter plates coated with either mouse monoclonal antibodies specific for the human Fas protein, both wild and mutant human p53, or rabbit polyclonal antibodies specific for the human p21/WAF1, and were incubated for 1 h (Fas) or 2 h (p53 or p21/WAF1) at room temperature. After removing unbound material by washing with PBS, horseradish peroxidase conjugated streptavidin was added to bind to the antibodies. Horseradish peroxidase catalyses the conversion of a chromogenic substrate (tetramethyl-benzidine) to a colored solution with a color intensity proportional to the amount of protein (p53, p21/ WAF1 or Fas) in the sample. The absorbance in each well was measured at 450 nm, and concentrations of p53, p21/WAF1 and Fas were determined by interpolating from standard curves obtained with known concentrations of proteins [24,25]. Assay for Western blotting The cells that were treated with 20 Ag/ml aloe-emodin for 24 or 48 h were lysed and protein concentrations were determined using Bio-Rad Protein Assay (Bio-Rad, Richmond, CA). For Western blotting, 50–100 Ag of a total cell lysate were subjected to SDS-PAGE. The proteins were transferredusing transfer buffer (50 mM Tris, 190 mM glycin, and 10% methanol) to PVDF membranes at 100 V for 2 h. The membranes were incubated with blocking buffer (50 mM Tris, 200 mM NaCl, 0.2% Tween 20, and 3% BSA) overnight at 4jC. After washing three times with washing buffer (blocking buffer without 3% BSA) for 10 min each, the blot was incubated with Bcl-2 or Bax antibody (Calbiochem, Cambridge, MA, USA) for 2–15 h followed by horseradish peroxidase-labeled secondary antibody (Amersham, USA) for 1 h. The membranes were washed again, and detection was performed using the enhanced chemiluminescence Western blotting detection system (Amersham, USA) [22].
Fig. 1. Effects of aloe-emodin on the proliferation inhibition of Hep G2 and Hep 3B cell lines. Adherent cells proliferation in 96-well plates (104 cells/well) were incubated with different concentrations (Ag/ml) for various lengths of time. Cell proliferation was determined by XTT assay. Results are expressed as the percentage of cell proliferation of the control at 0 h. The data shown are the mean from three independent experiments. Standard deviations are less than 10%. Fig. 2. Cell cycle analysis of Hep G2 cells following treated with or without 10 or 20 Ag/ml aloe-emodin for indicated time. Cells were fixed and stained with propidium iodide and then cell cycle distribution was analyzed by flow-cytometry. The percentage of the cells in each gate is indicated on the right-upper side.
Fig. 3. Cell cycle analysis of Hep 3B cells following treated with or without 10 or 20 Ag/ml aloe-emodin for indicated time. Cells were fixed and stained with propidium iodide and then cell cycle distribution was analyzed by flow-cytometry. The percentage of the cells in each gate is indicated on the right-upper side. P.-L. Kuo et al. / Life Sciences 71 (2002) 1879–1892 1884 Results Cell proliferation inhibition analysis Cell proliferation inhibition was determined by XTT assay. As shown in Fig. 1, aloe-emodin treatment induced proliferation inhibition in Hep G2 and Hep 3B. At 48 h, the maximal effect on proliferation inhibition was observed with 20 Ag/ml aloe-emodin, which inhibited proliferation in 70.98% of Hep G2 cells, and the IC50 value was 11.77 F 0.02 Ag/ml. However, in Hep 3B, the maximum effect on proliferation inhibition was observed with 20 Ag/ml aloe-eomdin, which inhibited proliferation only in 56.84% of the cells, with an IC50 value of 15.67 F 0.01 Ag/ml. Aloe-emodin demonstrated significant inhibitory effect in two examined cell lines, and the results were noted to have an obvious dose-dependent effect. Cell cycle distribution analysis To examine the mechanism responsible for cell proliferation inhibition, cell cycle distribution was evaluated using flow cytometry. Fig. 2 shows the mechanism responsible for cell proliferation inhibition
Apoptotic cell analysis by nucleosome ELISA assay Apoptosis is an active process of cell death. It is characterized by nuclear condensation, DNA fragmentation and blebbing of the plasma membrane. Activation of endonucleases during the process of apoptosis leads to the fragmentation of chromatin into oligonucleosomal fragments of multiples of f180 bp and the release of nucleosomes into the cytoplasm. By contrast, necrotic cell death occurs often with extensive tissue damage, resulting in an intense inflammatory response. The main character- istics of necrosis are swelling of organelles and cells. Later, the cell loses of membrane integrity and releases randomly digested DNA fragments. Thus, the quantification of the nucleosome in cytoplasm by a nucleosome ELISA assay that allows the detection and quantification of histones of nucleosomes can precisely measure apoptotic response [26]. Fig. 4 shows the time course of DNA fragmentation in continuous treatment with 10 or 20 Ag/ml aloeemodin. Both DNA fragmentation of Hep G2 and Hep 3B were exhibited at 6 h and maximized at 48 h after addition of aloe-emodin, and the effect of 20 Ag/ml was greater than at concentration 10 Ag/ml. Comparison of Fig. 1 and Fig. 4 indicates that both antiproliferative effect and DNA fragmentation had a turning point at 24 h, and both maximized at 48 h. This would be expected if the cell apoptosis in the two examined hepatoma cell lines.
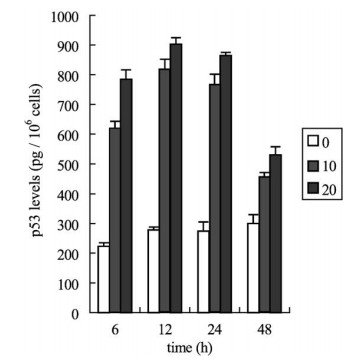
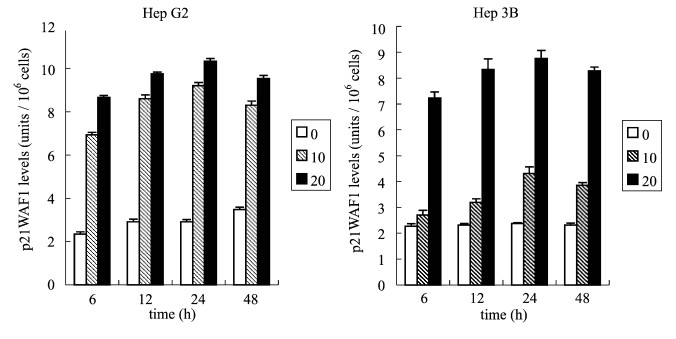
Fig. 5. Effects of aloe-emodin through the p53 protein expression of Hep G2 cell line. Hep G2 cells were treated with 10 or 20 Ag/ml aloe-emodin for 6, 12, 24, and 48 h. The level of p53 protein was measured by p53 pan ELISA kit. Each value is the mean F SD of three determinations. All values of the treated sample compared with the untreated (control) sample by Student’s t-test showed: p < 0.01. Aloe-emodin affects the expression of p53 and p21/WAF1 protein In the study of p53 protein expression, p53-positive Hep G2 was treated with 10 or 20 Ag/ml aloe- emodin. Our results indicated that the expression of p53 markedly increased after only 6 h incubation, and were maintained at a high level over 6–24 h of treatment with 10 and 20 Ag/ml aloe-emodin (Fig. 5). Furthermore, when Hep G2 was treated with 20 Ag/ml aloe-emodin, the induction of p53 expression was enhanced by a factor of 1.26, 1.09, 1.12, and 1.15 at 6, 12, 24, and 48 h, respectively, above that of the 10 Ag/ml-treated cells. These results show that the induction of p53 by aloe-emodin was enhanced following increasingly larger doses. To determine the effect of p21 of aloe-emodin and whether the expression of p21 is p53-dependent or independent, we assessed the expression of p21 in p53-positive Hep G2 and p53-deficient Hep 3B cells by WAF1 ELISA assay. The results in Fig. 6 show that in Hep G2 cells, the increase in p21 protein was apparent at 6 h, and the extent of p21 increased further at 24 h. In contrast, in Hep 3B treated with 10 Ag/ml aloe-emodin, the induction of p21 expression was only slightly enhanced. However, when used at a close IC50 concentration of 20 Ag/ml, aloe-emodin further enhanced p21 to reach 3.57, 3.62, 3.81 and 3.56 fold at 6, 12, 24 and 48 h, respectively, greater than that of untreated control cells.We suggest that aloe-emodin mediated apoptosis was through p21 activation in a p53-independent event in Hep 3B cells. Aloe-emodin affects the expression of Fas/APO1 receptor Because Fas receptor plays a trigger role in mediating apoposis, we asked whether Fas was involved in the cell death response induced by aloe-emodin. The level of Fas expression was measured after
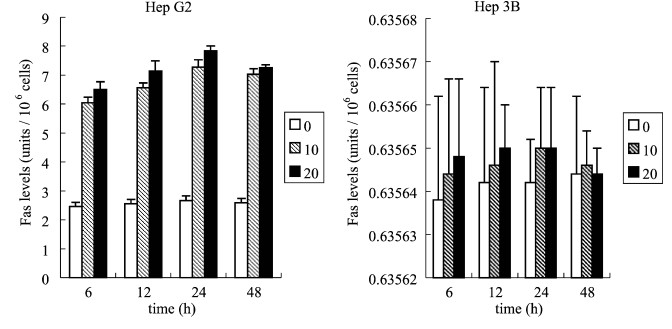
Fig. 6. Effects of aloe-emodin through the p21 protein expression of Hep G2 and Hep 3B cell lines. Hep G2 cells and Hep 3B cells were treated with 10 or 20 Ag/ml aloe-emodin for the indicated time. Then these cells were extracted and the expression of p21 was analyzed byWAF1 ELISA kit. The detailed protocol as described in ‘‘Methods’’. Each value is the mean F SD of three determinations. All values of the treated sample compared with the untreated (control) sample by Student’s t-test showed: p < 0.01 for Hep G2 treated with 10 and 20 Ag/ml aloe-emodin and for Hep 3B treated with 20 Ag/ml aloe-emodin, and p < 0.05 for Hep 3B treated with 10 Ag/ml aloe-emodin. treatment of Hep G2 and Hep 3B cells with 10 or 20 Ag/ml of aloe-emodin for 6, 12, 24 and 48 h. The results are shown in Fig. 7. In Hep G2 cells, both in treatment of 10 and 20 Ag/ml aloe-emodin, there was an increase in Fas within 6 h and peaked at 24 h. The fluctuation of the curve was similar to the curve of aloe-emodin-mediated p53 and p21 activation in Hep G2. By contrast, in Hep 3B cells, either in aloe-
Fig. 7. Effects of aloe-emodin through the Fas/APO1 expression of Hep G2 and Hep 3B cell lines. To survey Fas/APO1 concentration (units/106 cells) expression to treatment with 10 and 20 Ag/ml aloe-emodin for 6, 12, 24, and 48 h in Hep G2 and Hep 3B cell lines by Fas/APO1 ELISA kit. Each value is the mean F SD of three determinations. All values of the treated sample in Hep G2 compared with the untreated (control) sample by Student’s t-test showed: p < 0.01.
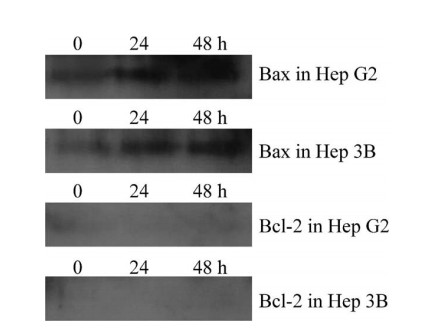
Fig. 8. The expression levels of Bax and Bcl-2 in Hep G2 and Hep 3B cell lines. Both Hep G2 and Hep 3B cells were exposed to 20 Ag/ml aloe-emodin for indicated time periods. Cells were harvested and subjected to Weastern bolt analysis with monoclonal antibodies against Bax and Bcl-2, respectively. emodin treated and untreated control cells, no significant change in the level of Fas expression at four time points was observed. Aloe-emodin effects on expression of Bcl-2 family Expression of Bcl-2 and Bax was investigated by Western analysis in untreated or treated Hep G2 and Hep 3B cells. In the case of pro-apoptotic Bax protein, both in Hep G2 and Hep 3B cells, 20 Ag/ml aloe-emodin increased Bax protein expression as compared with control cells at 24 and 48 h (Fig. 8). However, Bcl-2 protein was not detected in any of the hepatoma cells, including control and aloe-emodin treated cells. Discussion Aloe-emodin is one of major compositions of Rhei Rhizoma, used for the treatment of various liver diseases in traditional Chinese medicine. Previous study has reported that aloe-emodin can selectively inhibit human neuroectodermal tumor cell growth and lack of acute or chronic toxicity in animal model [10]. In this paper, we examined the antiproliferative effect in hepatoma cells and did further study of the molecular mechanistic basis of the anticancer properties of aloe-emodin. The results indicate that aloe-emodin inhibited cell growth by a pathway that was mediated by induction of apoptosis in Hep G2 and Hep 3B cells. In the p53-positive Hep G2 cell line, aloe-emodin treatment not only resulted in the accumulation of p53 and stimulated increased expression of CDK inhibitor p21, cell surface molecule Fas/APO1 and pro-apoptotic protein Bax, but also arrested the cell cycle in G1 phase. By contrast, aloe-emodin did not increase the expression of Fas/APO1 or inhibit cell cycle progression in p53-deficient Hep 3B cells. However, its antiproliferative mechanism is mediated by induction of p21 and Bax expression, resulting in apoptotic cell death. The cell growth inhibition, as well as the induced-apoptosis effect of aloe-emodin in Hep 3B cells, was less pronounced than in Hep G2 cells. Hep G2 cells have a normal tumor suppression gene p53, whereas in Hep 3B, a major protein of the p53 gene is deleted and accompanied by the absence of p53 transcriptions and p53 protein [27]. Many studies have demonstrated that chemoresistance of cancer cells during cancer therapy has been linked to the status of p53 [16–18,28]. This is supported by our finding of a reduced responsiveness of Hep 3B cells when compared to the responsiveness of Hep G2 cells in which p53 expression was induced by aloe-emodin. CDK-inhibitor p21, is a transcriptional target of p53. Previous study has indicated that p21 is required in p53-mediated cell cycle arrest and apoptosis [19]. Indeed, p21 plays an important role in the regulation of cell growth and differentiation. Development of sporadic tumors is generally associated with reduced expression of p21 [29,30], mainly as a result of loss the function of mutation p53 [30,31]. Moreover, p21 expression is directly related to terminal differentiation [30], and increased expression of p21 has been demonstrated to inhibit the proliferation of tumor cells in vitro and in vivo [32]. However, the role of p21 in the control of apoptosis is controversial. In some studies [33], authors have reported that increased p21 expression promoted apoptosis, whereas in other studies, authors have demonstrated that p21 is responsible for cell cycle arrest but not for apoptosis [32,34]. In our data, flow-cytometric analysis revealed that aloe-emodin could arrest Hep G2 cells in a G1 phase. This inhibition of cell-cycle progression might be associated with an altered expression of cell cycle relevant regulator, including p21 P.-L. Kuo et al. / Life Sciences 71 (2002) 1879–1892 1889and its upstream molecule, p53 [19]. Because the expression level of p21 was found to be significantly increased by treating Hep G2 cells with aloe-emodin, and the peak of p53 (at 12 h) occurred the earlier than peak of p21 (at 24 h), this suggests a possible role of p53 acting upstream of p21 in a signal transduction pathway initiated by aloe-emodin in Hep G2 cells. In contrast, in the Hep 3B cell line, the expression of p21 was largely increased when it was treated with 20 Ag/ml aloe-emodin at 24 h, and the data indicates that there was no evidence of cell cycle arrest in p21-expression Hep 3B cells. Furthermore, the appearance of DNA fragmentation was maintained at a high level within 24 h, and had only a slight change during 24–48 h. These results parallel the expression of p21 that peaked at 24 h and slightly decreased at 48 h. This implies that p21 failed to induce cell cycle arrest, but it might have triggered apoptotic cell death as the effect of okadaic acid and ceramide-induced apoptosis in cancer cells [35–37]. Upregulation of Fas/APO-1 receptor might be a major cause of apoptosis in chemotherapy. [11,12,38]. The action of p53 and its effect on the Fas/APO1 receptor/ligand system has demonstrated that p53 induces the expression of Fas/APO1 [19]. Our data showed that stimulation of the Fas receptor did occur only in Hep G2 cells with intact p53. In contrast, Hep 3B with deletion of p53 gene did not increase the Fas/APO1 receptor after aloe-emodin treatment. It is possible therefore, that the expression of the Fas/APO1 receptor is stimulated by p53 dependent transcriptional activity. The Bcl-2 family is the key regulator of apoptosis. It was not surprising that Bax expression increased in aloe-emodin treated Hep G2 cells. Wild-type p53 is known to be an upstream regulator of the Bax gene promoter that contains p53 binding sites, and can be directly activated by wild-type p53 [19]. The highest increase of Bax expression in Hep G2 after 48 h incubation of aloe-emodin may be the result of the increase of p53 accumulation that peaked earlier (after 24 h). Interestingly, in p53 negative Hep 3B cell line, Bax expression also increased after aloe-emodin treatment, and this result is in accordance with ceramide-induced apoptosis [35,36] that is associated with the upregulation of p21 and further increases of the expression level of Bax. Another member of the Bcl-2 family, Bcl-2 protein is an antagonist of apoptosis [39]. Bcl-2 in either Hep G2 or Hep 3 B cell line was undetectable in both drug-treated and untreated conditions, and this result was similar to a previous report which indicated that none of these cells expressed Bcl-2 [40]. Taken together, we have demonstrated that the naturally occurring plant anthroquinone, aloe-emodin, induced apoptosis in both human liver cancer cells Hep G2 and Hep 3B, but its apoptotic activities worked through different mechanisms. In the Hep G2 cell line, aloe-emodin increased accumulation of p53, thus further enhancing expression of p21/WAF1, Fas/APO1, and Bax. On the other hand, aloe- emodin induced apoptosis in Hep 3B was mediated by stimulation of p21/WAF1 that was p53- independent. However, we have clearly demonstrated that aloe-emodin could against examined hepatoma cell lines and it might be acted as a potential chemopreventive agent. References [1] Okuda K. Hepatocellular carcinoma: recent progress. Hepatology 1992;15(5):948–63. [2] Harris CC. Hepatocellular carcinogenesis: recent advances and speculations. Cancer Cells 1990;2(5):146–8. [3] Yu SZ. Primary prevention of hepatocellular carcinoma. Journal of Gastroenterology and Hepatology 1995;10(6):674–82. [4] Sheu JC. Molecular mechanism of hepatocarcinogenesis. Journal of Gastroenterology and Hepatology 1997;12(9–10):S309–13. P.-L. Kuo et al. / Life Sciences 71 (2002) 1879–1892 1890[5] Krumbiegel G, Schulz HU. Rhein and aloe-emodin kinetics from senna laxatives in man. Pharmacology 1993;47(Suppl 1): 120–4. [6] Agarwal SK, Singh SS, Verma S, Kumar S. Antifungal activity of anthraquinone derivatives from Rheum emodi. Journal of Ethnopharmacology 2000;72(1–2):43–6. [7] Hatano T, Uebayashi H, Ito H, Shiota S, Tsuchiya T, Yoshida T. Phenolic constituents of Cassia seeds and antibacterial effect of some naphthalenes and anhraquinones on methicillin-resistant Staphylococcus aureus. Chemical and Pharma- ceutical Bulletin 1999;47(8):1121–7. [8] Andersen DO, Weber ND, Wood SG, Hughes BG, Murray BK, North JA. In vitro virucidal activity of selected anthra- quinones and anthraquinone derivatives. Antiviral Research 1991;16(2):185–96. [9] Arosio B, Gagliano N, Fusaro LM, Parmeggiani L, Tagliabue J, Galetti P, De Castri D, Moscheni C, Annoni G. Aloe- Emodin quinone pretreatment reduces acute liver injury induced by carbon tetrachloride. Pharmacology and Toxicology 2000;87(5):229–33. [10] Pecere T, Gazzola MV, Mucignat C, Parolin C, Vecchia FD, Cavaggioni A, Basso G, Diaspro A, Salvato B, Carli M, Palu G. Aloe-emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors. Cancer Research 2000;60(11):2800–4. [11] Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation,and death. Cell 1994;76(6):959–62. [12] Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 1993;75(6):1169–78. [13] Galle PR, Hofmann WJ, Walczak H, Schaller H, Otto G, Stremmel W, Krammer PH, Runkel L. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. The Journal of Experimental Medicine 1995;182(5):1223–30. [14] Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature 1993;364(6440):806–9. [15] Jiang S, Song MJ, Shin EC, Lee MO, Kim SJ, Park JH. Apoptosis in human hepatoma cell lines by chemotherapeutic drugs via Fas-dependent and Fas-independent pathways. Hepatology 1999;29(1):101–10. [16] Fisher DE. Apoptosis in cancer therapy: crossing the threshold. Cell 1994;78(4):539–42. [17] Falette N, Paperin MP, Treilleux I, Gratadour AC, Peloux N, Mignotte H, Tooke N, Lofman E, Inganas M, Bremond A, Ozturk M, Puisieux A. Prognostic value of P53 gene mutations in a large series of node-negative breast cancer patients. Cancer Research 1998;58(7):451–5. [18] Brown JM, Wouters BG. Apoptosis, p53, and tumor cell sensitivity to anticancer agents. Cancer Research 1999; 59(7):1391–9. [19] May P, May E. Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene 1999; 18(53):7621–36. [20] Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. Journal of Immunological Methods 1991;142(2):257–65. [21] Goodwin CJ, Holt SJ, Downes S, Marshall NJ. Microculture tetrazolium assays: a comparison between two new tetra- zolium salts, XTT and MTS. Journal of Immunological Methods 1995;179(1):95–103. [22] Shirin H, Sordillo EM, Oh SH, Yamamoto H, Delohery T, Weinstein IB, Moss SF. Helicobacter pylori inhibits the G1 to S transition in AGS gastric epithelial cells. Cancer Research 1999;59(10):2277–81. [23] Kikuchi S, Hiraide H, Tamakuma S, Yamamoto M. Expression of wild-type p53 tumor suppressor gene and its possible involvement in the apoptosis of thyroid tumors. The Japanese Journal of Surgery 1997;27(3):226–33. [24] Mediavilla MD, Cos S, Sanchez-Barcelo EJ. Melatonin increases p53 and p21WAF1 expression in MCF-7 human breast cancer cells in vitro. Life Sciences 1999;65(4):415–20. [25] Tendler Y, Weisinger G, Coleman R, Diamond E, Lischinsky S, Kerner H, Rotter V, Zinder O. Tissue-specific p53 expression in the nervous system. Brain Research. Molecular Brain Research 1999;72(1):40–6. [26] Salgame P, Varadhachary AS, Primiano LL, Fincke JE, Muller S, Monestier M. An ELISA for detection of apoptosis. Nucleic Acids Research 1997;25(3):680–1. [27] Puisieux A, Galvin K, Troalen F, Bressac B, Marcais C, Galun E, Ponchel F, Yakicier C, Ji J, Ozturk M. Retinoblastoma and p53 tumor suppressor genes in human hepatoma cell lines. The FASEB Journal 1993;7(14):1407–13. [28] Friedman SL, Shaulian E, Littlewood T, Resnitzky D, Oren M. Resistance to p53-mediated growth arrest and apoptosis in Hep 3B hepatoma cells. Oncogene 1997;15(1):63–70. [29] Sinicrope FA, Roddey G, Lemoine M, Ruan S, Stephens LC, Frazier ML, Shen Y, Zhang W. Loss of p21WAF1/Cip1 P.-L. Kuo et al. / Life Sciences 71 (2002) 1879–1892 1891protein expression accompanies progression of sporadic colorectal neoplasms but not hereditary nonpolyposis colorectal cancers. Clinical Cancer Research 1998;4(5):1251–61. [30] Doglioni C, Pelosio P, Laurino L, Macri E, Meggiolaro E, Favretti F, Barbareschi M. p21/WAF1/CIP1 expression in normal mucosa and in adenomas and adenocarcinomas of the colon: its relationship with differentiation. The Journal of Pathology 1996;179(3):248–53. [31] Rodrigues NR, Rowan A, Smith ME, Kerr IB, Bodmer WF, Gannon JV, Lane DP. p53 mutations in colorectal cancer. Proc Natl Acad Sci U S A 1990;87(19):7555–9. [32] Yang ZY, Perkins ND, Ohno T, Nabel EG, Nabel GJ. The p21 cyclin-dependent kinase inhibitor suppresses tumorigenicity in vivo. Nature Medicine 1995;1(10):1052–6. [33] Chinery R, Brockman JA, Peeler MO, Shyr Y, Beauchamp RD, Coffey RJ. Antioxidants enhance the cytotoxicity of chemotherapeutic agents in colorectal cancer: a p53-independent induction of p21WAF1/CIP1 via C/EBPbeta. Nature Medicine 1997;3(11):1233–41. [34] Wenzel U, Kuntz S, Brendel MD, Daniel H. Dietary flavone is a potent apoptosis inducer in human colon carcinoma cells. Cancer Research 2000;60(14):3823–31. [35] Sheikh MS, Garcia M, Zhan Q, Liu Y, Fornace AJ. Cell cycle-independent regulation of p21Waf1/Cip1 and retinoblas- toma protein during okadaic acid-induced apoptosis is coupled with induction of Bax protein in human breast carcinoma cells. Cell Growth and Differentiation 1996;7(12):1599–607. [36] Kang KH, Kim WH, Choi KH. p21 promotes ceramide-induced apoptosis and antagonizes the antideath effect of Bcl-2 in human hepatocarcinoma cells. Experimental Cell Research 1999;253(2):403–12. [37] Oh WJ, KimWH, Kang KH, Kim TY, Kim MY, Choi KH. Induction of p21 during ceramide-mediated apoptosis in human hepatocarcinoma cells. Cancer Letters 1998;129(2):215–22. [38] Muller M, Strand S, Hug H, Heinemann EM, Walczak H, Hofmann WJ, Stremmel W, Krammer PH, Galle PR. Drug-induced apoptosis in hepatoma cells is mediated by the CD95 (APO-1/Fas) receptor/ligand system and involves activation of wild-type p53. The Journal of Clinical Investigation 1997;99(3):403–13. [39] Antonsson B, Martinou JC. The Bcl-2 protein family. Experimental Cell Research 2000;256(1):50–7. [40] Takehara T, Liu X, Fujimoto J, Friedman SL, Takahashi H. Expression and role of Bcl-xL in human hepatocellular carcinomas. Hepatology 2001;34(1):55–61. P.-L. Kuo et al. / Life Sciences 71 (2002) 1879–1892 1892 |







 The antiproliferative activity of aloe-emodin is through p53-dependent and p21-dependent apoptotic pathway in human hepatoma cell lines
The antiproliferative activity of aloe-emodin is through p53-dependent and p21-dependent apoptotic pathway in human hepatoma cell lines 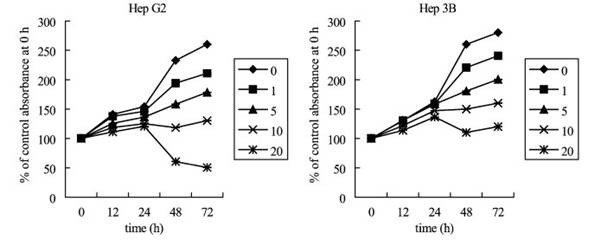
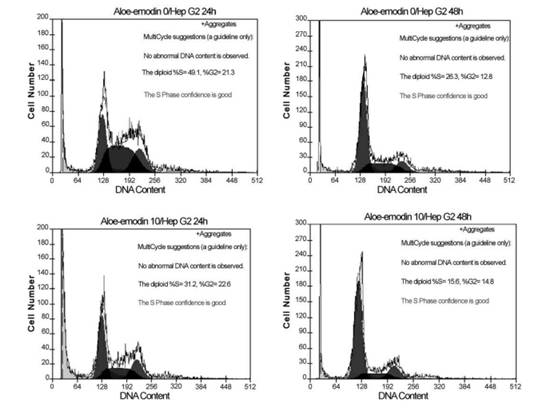

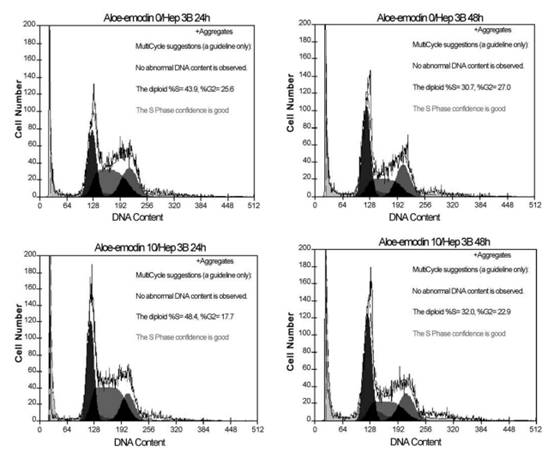
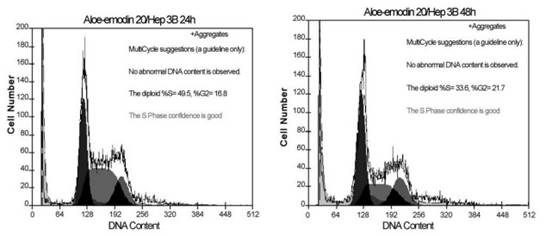
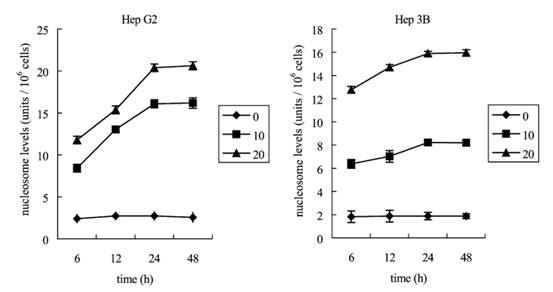 Fig. 4. Kinetics of aloe-emodin-induced apoptotic cell death in Hep G2 and Hep 3B cells. Hep G2 and Hep 3B cells were cultured with 10 or 20 Ag/ml aloe-emodin for 6, 12, 24 and 48 h. Cells were harvested and lysed with lysis buffer. Cell lysates that contain cytoplasmic oligonucleosomes of apoptotic cells were analyzed in the Nucleosome ELISA. Each value is the mean F SD of three determinations. All values of the treated sample compared with the untreated (control) sample by Student’s t-test showed: p < 0.01. P.-L. Kuo et al. / Life Sciences 71 (2002) 1879–1892 1885in aloe-emodin-treated Hep G2 cells. Aloe-emodin caused an accumulation of cells in G1 phase at both 10 and 20 Ag/ml. This effect was pronounced at 20 Ag/ml, and the inhibition of cell cycle progression by aloe-emodin was in a time-dependent manner. As shown in Fig. 3, regardless of changes of dosage or time, no evidence of significant cell cycle arrest was detected.
Fig. 4. Kinetics of aloe-emodin-induced apoptotic cell death in Hep G2 and Hep 3B cells. Hep G2 and Hep 3B cells were cultured with 10 or 20 Ag/ml aloe-emodin for 6, 12, 24 and 48 h. Cells were harvested and lysed with lysis buffer. Cell lysates that contain cytoplasmic oligonucleosomes of apoptotic cells were analyzed in the Nucleosome ELISA. Each value is the mean F SD of three determinations. All values of the treated sample compared with the untreated (control) sample by Student’s t-test showed: p < 0.01. P.-L. Kuo et al. / Life Sciences 71 (2002) 1879–1892 1885in aloe-emodin-treated Hep G2 cells. Aloe-emodin caused an accumulation of cells in G1 phase at both 10 and 20 Ag/ml. This effect was pronounced at 20 Ag/ml, and the inhibition of cell cycle progression by aloe-emodin was in a time-dependent manner. As shown in Fig. 3, regardless of changes of dosage or time, no evidence of significant cell cycle arrest was detected.