| Emodin protects rat liver from CCl4-induced fibrogenesis via inhibition of hepatic stellate cells activation |
| 发布时间:2012-07-20 信息来源:admin 发布人:admin 点击次数:5995 |
Abstract AIM: To investigate the role of emodin in protecting the l iver against f ibrogenesis caused by carbon tetrachloride (CCl4) in rats and to further explore the underlying mechanisms. METHODS: Rat models of experimental hepatic fibrosis were established by injection with CCl4;the treated rats received emodin via oral administration at a dosage of 20 mg/kg twice a week at the same time. Rats injected with olive oil served as a normal group. Histopathological changes were observed by hematoxylin and eosin staining. The activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in serum and hepatic hydroxyproline content were assayed by biochemical analyses. The mRNA and protein relevant to hepatic stellate cell (HSC) activation in the liver were assessed using real-time reverse transcription-polymerase chain reaction (RT-PCR), immunohistochemistry, western blotting and enzyme-linked immunosorbent assay. RESULTS: The degree of hepatic fibrosis increased markedly in the CCl4 group compared to the normal group (P < 0.01), and decreased markedly in the emodin group compared to the CCl4 group according to METAVIR scale ( P < 0.01) compared with those in the normal control group (51.02 ± 10.64 IU/L and 132.28 ± 18.14 IU/L). The activities of serum ALT and AST were significantly higher in rats injected with CCl4 (289.25 ± 68.84 IU/L and 423.89 ± 35.67 IU/L, both P < 0.05). The activities of serum ALT and AST were significantly reduced by administration of emodin (176.34 ± 47.29 IU/L and 226.1 ± 44.52 IU/L, both P < 0.05). Compared with the normal controls (54.53 ± 13.46 mg/g), hepatic hydroxyproline content was significantly higher in rats injected with CCl4 (120.27 ± 28.47 mg/g, P < 0.05). Hepatic hydroxyproline content was significantly reduced in the rats treated with emodin at 20 mg/kg (71.25 ± 17.02 mg/g, P < 0.05). Emodin significantly protected the liver from injury by reducing serum AST and ALT activities and reducing hepatic hydroxyproline content. The mRNA levels of transforming growth factor-β1 (TGF-β1), Smad4 and α-SMA in liver tissues were significantly down-regulated in SD rats that received emodin treatment. Furthermore, significant down-regulation of serum TGF-β1 protein levels and protein expression of Smad4 and α-SMA in liver tissues was also observed in the rats. Emodin inhibited HSC activation by reducing the abundance of TGF-β1 and Smad4. CONCLUSION: Emodin protects the rat liver from CCl4-induced fibrogenesis by inhibiting HSC activation. Emodin might be a therapeutic antifibrotic agent for the treatment of hepatic fibrosis. © 2009 The WJG Press and Baishideng. All rights reserved. Key words: Emodin; Hepatic fibrosis; Transforming growth factor-β1; Smad4; Hepatic stellate cell; α-smooth muscle actin Peer reviewer: Andrew D Clouston, Associate Professor, Histopath Laboratories, Suite 4, Level 9, Strathfield Plaza, Strathfield, Sydney, 2135, Australia Dong MX, Jia Y, Zhang YB, Li CC, Geng YT, Zhou L, Li XY, Liu JC, Niu YC. Emodin protects rat liver from CCl4-induced fibrogenesis via inhibition of hepatic stellate cells activation. World J Gastroenterol 2009; 15(38): 4753-4762 Available from: URL: http://www.wjgnet.com/1007-9327/15/4753.asp DOI: http://dx.doi.org/10.3748/wjg.15.4753 INTRODUCTION Hepatic fibrosis occurs in advanced l iver disease, where normal hepatic tissue is replaced with collagen-rich extracellular matrix (ECM) and, if left untreated, results in cirrhosis. Several studies have shown that hepatic fibrosis is a reversible disease, therefore an effective treatment would probably prevent or reverse the fibrotic process in the liver[1,2]. Transforming growth factor β1 (TGF-β1) is one of the strongest profibrotic cytokines[3,4], and TGF-β1/Smad signaling is the cardinal signal transduction pathway involved in fibrosis which has been verified by several related studies[5]. The down regulation of TGF-β1 expression and modulation of TGF-β/Smad signaling may be effective in preventing liver fibrosis[6]. In the last decade, advances in the understanding of genes promoting hepatic stellate cell (HSC) activation are impressive[7]. However, there are few breakthroughs in therapeutic intervention of hepatic fibrogenesis. Efficient and well-tolerated antifibrotic drugs are lacking and current treatment of hepatic fibrosis is limited to withdrawal of the noxious agent[8]. Therefore, research identifying innocuous antifibrotic agents is of high priority and urgently needed. Emodin (1,3,8-trihydroxy-6-methylanthraquinone), isolated from the rhizome of the Giant Knotweed Rhizome, has been used for centuries in Asia as a treatment for inflammation, gastrointestinal, pulmonary, and liver disorders. Emodin is regarded as the most active constituent in Giant Knotweed Rhizome and exerts many potent biological effects[9,10], such as anticancer[11], antimicrobial[12], and anti-inflammatory effects[13]. Several studies have revealed that emodin is efficacious in the management of hepatic fibrosis[14,15]. However, the mechanisms underlying remain to be elucidated. The current study evaluates the in vivo role of emodin in the protection of the liver from fibrogenesis caused by carbon tetrachloride (CCl4) in a rat model and further explores the underlying mechanisms. We hypothesize that emodin might protect the liver from CCl4-induced fibrogenesis by inhibiting activation of HSC via modulating TGF-β1/Smad signaling pathways. Results in this study support our hypothesis and provide novel insight into the mechanisms of emodin in the protection of the liver. MATERIALS AND METHODS Animals This study was approved by the Animal Care and Use Committee of Qiqihar Medical University. A total of 50 pathogen-free male Sprague-Dawley (SD) rats (weight range: 200-240 g) were employed in the study. The animals were obtained from the Beijing Vital River Experimental Animals Technology (Beijing, China), and were housed in sterile cages under laminar airflow hoods in a specific pathogen-free room with a 12 h light and 12 h dark schedule and fed autoclaved chow and water ad libitum. The animals were weighed every 7 d for the adjustment of the CCl4 and emodin doses. Emodin were purchased from Xi’an Sino-Herb Bio-Technology CO., LTD (Purity: 98% by HPLC). Establishment of a rat model with hepatic fibrogenesis caused by CCl4 The rat model was established using the method originally described by Proctor et al[16]and since used by many others[17], with minor modifications. Fifty male SD rats were randomLy divided into three groups: the normal control (n = 10) in which rats were not administrated CCl4 or emodin, but they were injected with olive oil and orally given sodium carboxymethylcellulose (CMC); the CCl4 group (n = 20) in which rats were subcutaneously injected with CCl4, without emodin treatment; the emodin group in which rats were injected with CCl4 and treated with emodin at 20 mg/kg. Rats from the emodin group and the CCl4 group were subcutaneously injected with a mixture of 40% CCl4 (a mixture of pure CCl4 and sterile olive oil) at 200 µL/100 g body weight twice weekly for 12 wk. Emodin was dissolved in 0.5% sodium CMC and given once daily by gavage at 20 mg/kg. The rats in the normal group were similarly handled, including subcutaneous injections with the same volume of olive oil and oral administration of the same volume of CMC without emodin. At the end of the experiment, the survivors in the normal group, CCl4 group and emodin group were 10/10, 9/20 and 11/20, respectively. Forty-eight hours after the last CCl4 injection, rats were sacrificed after being anesthetized by i.p. pentobarbital (50 mg/kg). A small portion of the liver was removed for hematoxylin and eosin (HE) staining and immunohistochemistry (IHC) studies by fixation with 10% formalin. The remaining liver was cut in pieces and rapidly frozen with liquid nitrogen for extraction of total RNA and protein. Blood was collected directly from the rats when they were sacrificed. Serum was separated by centrifugation within 1 h of blood collection and stored at -20℃ until analyzed. Light microscopy Midsections of the liver lobe a few mm thick were taken from each rat and processed for observation by light microscopy. The process involved fixing the tissue specimen in 10% neutral buffered formalin solution, preparing the block in paraffin, cutting into 5-6 µm thick sections, and staining the sections with HE. The sections were scanned and analyzed by a pathologist who was blinded to the different treatments in the experiment. The histological changes were measured on HE stained sections. Lobular inflammatory activity and severity of liver steatosis were determined according to the criteria of the Chinese Medical Association Committee of Fatty Liver Disease in 2006 and Nouchi et al[18,19]. Steatosis was graded on the basis of the extent of parenchyma involved as Grade 0, no hepatocytes were involved; Grade 1, < 30% of hepatocytes were involved; Grade 2, 30% to 50% of hepatocytes were involved; Grade 3, 51% to 75% of hepatocytes were involved; Grade 4, > 75% of hepatocytes were involved. Inflammation was graded as Grade 1, focal collections of mononuclear inflammatory cells; Grade 2, diffuse infiltrates of mononuclear inflammatory cells; Grade 3, focal collections of polymorphonuclear cells in addition to mononuclear cell infiltrates; and Grade 4, diffuse infiltrates of polymorphonuclear cells in the parenchymal area or lobular area. The stage of liver fibrosis was graded with the METAVIR scale [20], which grades fibrosis on a five-point scale: Grade 0, no fibrosis; Grade 1, portal fibrosis without septa; Grade 2, portal fibrosis with a few septa; Grade 3, numerous septa without cirrhosis; and Grade 4, cirrhosis. Biochemical parameters Activities of alanine transaminase (ALT) and aspartate aminotransferase (AST) in serum were measured by routine laboratory methods using a 7170-automatic biochemistry analyzer (Tokyo, Japan). Determination of the hepatic hydroxyproline content The hydroxyproline kit was purchased from Nanjing Jiancheng Bioengineering Research Institute (Nanjing, China). The content of hepatic hydroxyproline was determined by using the hydroxyproline kit following the protocol provided by the manufacturer. Results were expressed as micrograms of hydroxyproline per gram of hepatic tissue. Enzyme-linked immunosorbent assay (ELISA) The TGF-β1 ELISA kit was obtained from Boster Biotechnology Co. Ltd. (Wuhan, China). The levels of TGF-β1 in serum were determined by using the TGF-β1 ELISA kit according to the manufacturer’s protocol. In brief, 100 µL of a serum sample was added to each well of the plate,followed by incubation for 2 h at 37℃. A Working Detector (100 µL; Boster Biotechnology Co. Ltd) was loaded into each well, and theplate was incubated for an additional 1 h at room temperature (RT) before the addition of substrate solution (100 µL; Boster Biotechnology Co. Ltd). The reaction was stopped by adding stop solution (1 drop; Boster Biotechnology Co. Ltd).The absorbance was read at 492 nm using a Microplate reader (LabSystems Multiskan Ascent 354, Finland). Calculation of the concentrationsof TGF-β1 was performed in a log-log linear regression according to the instructions in the protocol. IHC analysis Liver tissues were fixed in 10% neutral buffered formalin solution, embedded in paraffin, and stained for routine histology. The sections were incubated at 4℃ overnight with primary antibody (Boster Biotechnology, Wuhan, China) in concentrations of 1:100 (Smad4) and 1:200 (alpha-SMA). As a secondary antibody, horseradish peroxidase-conjugated immunoglobul in G (Boster Biotechnology), was used for 30 min at 37℃. After furtherwashing with Tris-buffered saline, sections were incubated with complex/horseradish peroxidase (1:200 dilution)for 30 min at 37℃. Immunolocalization was performed byimmersion in 0.05% 3,3-diaminobenzidine tetrahydrochloride as chromagen. Slides were counter-stained with hematoxyl in before dehydration and mounting. Incubation without the primary antibody was performed as a control for the background staining. Histological evaluation was performed by a pathologist who was blind to the pharmacological characteristics of the drugs. RNA isolation and real-time reverse transcription polymerase chain reaction (RT-PCR) Total RNA was extracted from 100 mg of frozen liver tissues using RNAiso Reagent kit (Takara Biotechnology, Dalian, China) according to the protocol provided by the manufacturer. cDNA was synthesized with SYBR ExScript™ RT-PCR kit (Takara Biotechnology, Dalian, China) according to the protocol provided by the manufacturer. Reverse transcription was carried out as follows: 42℃ for 15 min, 95℃ for 2 min (one cycle). cDNA was stored at -20℃ for PCR. Real-time PCR was performed in 50 µL of reaction solution containing 2 × SYBR Premix Ex Taq polymerase, deoxynucleoside triphosphates, ROX Reverence Dye and the corresponding primers. The cycles for PCR were as follows: 1 cycle of 95℃ for 10 s, 40 cycles of 5 s at 95℃, 5 s at 60℃, 31 s at 60℃ and a final 7 min at 72℃. Melting curve analysis was always included to validate the specificity of the PCR products. Serial cDNA dilution curves were produced to calculate the amplification efficiency for all genes. A graph of threshold cycle (Ct) versus log 10 relative copy number of the sample from a dilution series was produced. The slope of the curve was used to determine the amplification efficiency. Reactions were performed in an ABI7300 Real-time PCR system (Applied Biosystems, CA) and threshold cycle (Ct) data were collected using the Sequence Detection Software version 1.2.3 (Applied Biosystems, CA). GAPDH was used as an internal control. mRNA -fold change relative to GAPDH was calculated with the comparative Ct method of 2-ΔΔCt[21]. The following primers were used. 5-GACAACTTTGGCATCGTGGA-3 (sense) and 5-ATGCAGGGATGATGTTCTGG-3 (antisense) for the GAPDH gene; 5-CCTGATGCTTCACTGTTCTGCAA-3 (sense) and 5-CAACTGCACGGTTTCCGTTATTC-3(antisense) for the Smad4 gene; 5-TATAGCAACAATTCCTGGCG-3 (sense) and 5-TGCTGTCACAGGAGCAGTG-3 (antisense) for the TGF-β1 gene; 5-CCGAGATCTCACCGACTACC-3 (sense) and 5-TCCAGAGCGACATAGCACAG-3 (antisense) for the α-SMA gene. mRNA levels were expressed as -fold changes after normalization with GAPDH. All tests were done in triplicate to ensure reproducibility. Western blotting Cytoplasm proteins were isolated from 120 mg of frozen l iver t issues using a Cytoplasmic Protein Extraction kit (Beyotime Biotechnology, Haimen, China) according to the protocol provided by the manufacturer. Protein concentrations were determined using the BCA Protein Assay kit according to the protocol provided by the manufacturer (Beyotime Biotechnology, Haimen, China). 100 µL of supernatant was added to an equal volume of 2 × SDS sample buffer and boi led for 5 min at 100℃. The samples were then stored at -80℃ until analyzed. The electrophoretic mobility of the proteins analyzed in this study was determined by SDS-polyacrylamide gel electrophoresis using 15% acrylamide concentrations. After electrophoresis, the proteins were transferred electrophoretically to a nitrocellulose filter membrane that was then blocked for 4 h in a solution of 8% nonfat dry milk in Tris-buffered saline containing 0.1% tween (pH 7.6) at RT. The membrane was then incubated overnight at 4℃ with Smad4 antibody and GAPDH antibody which are represented on Western blotting by two distinct bands at 65 and 36 kDa. Bands were washed four times, after which they were incubated with Horseradish Peroxidase Labeled Anti-Mouse IgG (Medical Biological Laboratory, Nagoya, Japan) for 2 h and again washed four times. The blots were developed using an ECL Western blotting kit as recommended by the manufacturer. GAPDH was probed as an internal control. GAPDH was used to confirm that an equal amount of protein was loaded in each lane. Band intensities were determined using an AlphaImager™ 2200 using the SpotDenso function of AlphaEaseFC™ Software version 3.1.2 (Witec, Littau, Switzerland). Statistical analysis All determinations were repeated three times, and results are expressed as the mean ± SD. ANOVA was used to evaluate the difference among multiple groups followed by a post hoc test (Student-Newman-Keuls) for quantitative data, and RIDIT test was used for statistical analysis of qualitative data. The data were analyzed using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA), and P < 0.05 was considered statistically significant. RESULTS Emodin protected the liver against CCl4-induced injury and suppressed hepatic fibrogenesis in the rat model The effects of emodin on the protection of the liver from injury and fibrogenesis were initially evaluated by histological analyses. Representative views of liver sections are shown in Figure 1A. As shown in tissue sections stained with HE, compared with sections from livers in the vehicle controls (normal group), CCl4 caused prominent hepatic steatosis, necrosis, and formation of regenerative nodules and fibrotic septa between the nodules (CCl4 group). Oral administration of emodin dai ly for 12 wk improved the state of steatosis with a significant reduction in the number of macro- and microvesicular steatosis lesions, and it apparently suppressed hepatic fibrogenesis by reducing the thickness of bridging fibrotic septa (emodin group).
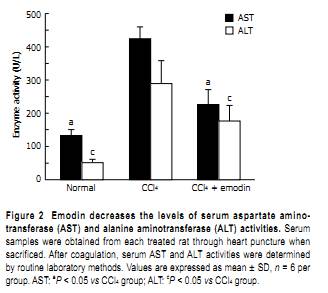
According to METAVIR scale, the degree of hepatic fibrosis increased markedly in the CCl4 group compared to the normal group, and decreased markedly in the emodin group compared to the CCl4 group (P < 0.01, Table 1). Taken together, emodin reduced hepatic fibrogenesis caused by chronic CCl4 intoxication. Emodin reduced the content of hepatic hydroxyproline in the CCl4 rat model The efficacy of treatment with emodin on protection of the liver from fibrogenesis was further evaluated by using a quantitative method to determine the content of hepatic hydroxyproline in the rat model. Compared with the normal controls (54.53 ± 13.46 mg/g), the hepatic hydroxyproline content was significantly higher in rats injected with CCl4 (120.27 ± 28.47 mg/g, P < 0.05). The hepatic hydroxyproline content was significantly reduced in rats treated with emodin at 20 mg/kg (71.25 ± 17.02 mg/g, P < 0.05). Emodin suppresses serum activities of ALT and AST in the CCl4 rat model Biochemical analyses of serum enzymes were performed to verify the role of emodin in the protection of the liver from injury. As shown in Figure 2, compared with those in the normal controls (51.02 ± 10.64 IU/L and 132.28 ± 18.14 IU/L), the activities of serum ALT and AST were significantly higher in rats injected with CCl4 (289.25 ± 68.84 IU/L and 423.89 ± 35.67 IU/L). The activities of serum ALT and AST were significantly reduced by administration of emodin (176.34 ± 47.29 IU/L and 226.1 ± 44.52 IU/L). These results demonstrated that emodin protected the liver against CCl4-induced injury. Emodin reduces HSC activation in the liver in the CCl4 rat model IHC and real-time PCR experiments were performed to further evaluate the impact of emodin on regulating
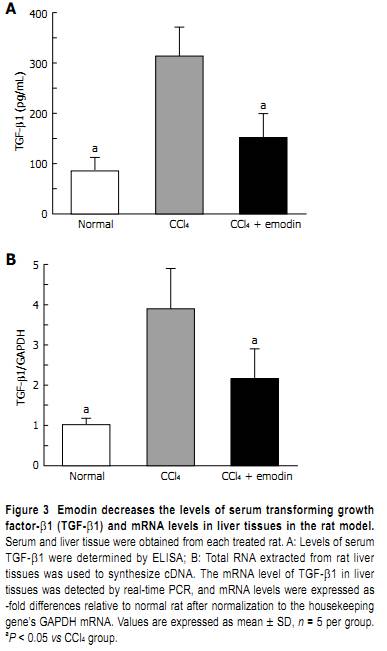
the expression of α-SMA, the marker of activated HSC. Liver sections from each group were immunolabeled with antibodies against α-SMA. As shown in Figure 1B, as expected, few cells in the liver sections from the normal group were recognized by antibodies against α-SMA, suggesting few activated HSC in the normal livers in the vehicle control rats. Administration of CCl4 caused a significant increase in the number of cells recognized by antibodies against α-SMA. Emodin treatment significantly reduced the number of cel ls labeled with α-SMA antibodies, suggesting that emodin might suppress HSC activation in the rat model. The comparative Ct method of 2-ΔΔCt and IHC evaluation result showed that protein and mRNA levels of α-SMA in liver tissues from normal control rats were 8.88 ± 1.26 and 1.01 ± 0.19, respectively while those in the CCl4 group were 21.97 ± 1.68 and 3.52 ± 0.60, respectively. Treatment of rats with emodin during CCl4 exposure largely increased expression of α-SMA and resulted in protein and mRNA levels of 14.61 ± 1.67 and 2.46 ± 0.91, respectively (Figure 1C and D). Emodin reduces the concentration of TGF-β1 in serum and mRNA levels in liver tissues TGF-β1 is the major profibrogenic factor during hepatic fibrogenesis. We examined the effect of emodin on the concentration of TGF-β1 in serum and mRNA levels in liver tissues of the rat model by ELISA and real-time PCR. As shown in Figure 3, compared with those in the normal group (84.89 ± 27.14 pg/mL and 1.01 ± 0.16, respectively), the levels of TGF-β1 in serum (Figure 3A) and mRNA levels of TGF-β1 in liver tissues (Figure 3B) were dramatically increased in the CCl4 group (313.40 ± 57.75 pg/mL and 3.89 ± 1.00, respectively, both P < 0.05 vs normal group). The levels of TGF-β1 in serum and mRNA levels of TGF-β1 in liver tissues were significantly reduced in the emodin group (151 ± 47.64 pg/mL and
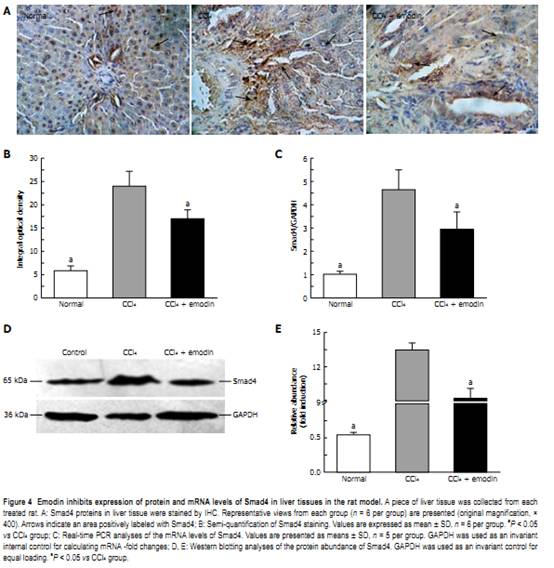
2.16 ± 0.73, respectively, both P < 0.05 vs CCl4 group). Although these was still higher than those of the normal group, these data indicated that emodin significantly reduced the levels of TGF-β1 in serum and mRNA levels in liver tissues in the rat model, which might result in the inhibition of HSC activation stimulated by CCl4. Emodin down-regulates the protein and mRNA levels of Smad4 in liver tissues of the CCl4 rat model Because TGF-β1 signals within the cell through Smad is involved in fibrosis, the effects of emodin on mRNA and protein levels of Smad4 in liver tissues were demonstrated by real-time PCR (Figure 4C), Western blotting (Figure 4D and E), and IHC analyses (Figure 4A and B). Experiments revealed that exposure of rats to CCl4 significantly increased mRNA and protein levels of Smad4 in liver tissues from 1.00 ± 0.13, 0.54 ± 0.04 and 5.78 ± 1.05, respectively, in the normal group to 4.63 ± 0.86, 13.44 ± 0.64 and 23.95 ± 3.23, respectively, in the CCl4 group. In contrast, protein and mRNA levels of Smad4 in liver tissues from rats treated with emodin during CCl4 exposure were attenuated and were 2.94 ± 0.74, 9.25 ± 0.84 and 17.00 ± 1.88, respectively. Treatment of rats with emodin during CCl4 exposure blunted the increase in protein and mRNA levels of Smad4 significantly. DISCUSSION In the present study, we confirmed that emodin protects the rat liver from CCl4-induced injury and fibrogenesis. The mechanism for this protective effect may relate to the fact that emodin efficiently inhibits HSC activation in vivo. Hepatic fibrosis, which may lead to cirrhosis, is associated with most chronic liver diseases[22]. Hepatic fibrosis is thought to be a reversible disease, however, there is no satisfactory method in clinical practice to reverse the pathological process yet[23]. Several drugs, including antisense TGF-β1 receptors, cytokines[24], antioxidants, chemical drugs[25], soluble type Ⅱ receptor of TGF-β1, and TGF-β1 antibodies[26] have been used in research work to block experimental hepatic fibrosis, but
their effects were not as prosperous as we had expected. Some traditional Chinese drugs have been found effective in preventing fibrogenesis and other causes of chronic liver injury[27,28], and this helps to develop a more hopeful future in controlling liver fibrosis and cirrhosis. Emodin is a main active monomer isolated from Giant Knotweed Rhizome, which is widely used in traditional Chinese herb treatment of liver cirrhosis[29]. It is easy to extract, isolate and identify emodin, so it shows excellent prospects in the development of some new drugs for treating hepatic fibrosis. CCl4, a highly toxic chemical agent, causes hepatic injury including hepatocytic necrosis, steatosis, and inflammation. Research for establishing a model of liver fibrosis with CCl4 began in 1936. Since then many methods to establish a model of liver fibrosis have been tried[30]. Among them, hepatic fibrosis caused by CCl4 has been extensively used in experimental models in rats because hepatic responses in rats to chronic CCl4 stimulation are shown to be superficially similar to human cirrhosis[31]. Hepatocyte damage is the initial factor of hepatic fibrogenesis and activities of ALT and AST in serum are the most commonly used biochemical markers of liver injuries[32]. Hydroxyproline is an amino acid found
almost exclusively in collagens. Determination of the content of hydroxyproline in liver tissue is regarded as a good method to quantify fibrosis and to evaluate the effectiveness of new potentially antifibroticagents. In this study, the method of subcutaneously injecting CCl4 was used to establish the model of liver fibrosis. Histological analysis showed CCl4 caused prominent hepatic steatosis, necrosis, and formation of regenerative nodules and fibrotic septa between the nodules. Biochemical assay showed serum ALT activities, serum AST activities, and content of hepatic hydroxyproline were markedly increased in rats injected with CCl4 for 12 wk, which are consistent with the histological observations. Our results suggest that oral administration of edomin daily for 12 wk improved the state of steatosis with a significant reduction in the number of macro- and microvesicular steatosis, and it also apparently suppressed hepatic fibrogenesis by reducing the thickness of bridging fibrotic septa. Emodin could decrease the scores of hepatic fibrosis grading, inhibit the ALT and AST activities in serum and reduced the content of hepatic hydroxyproline. All results confirm that emodin protected the liver from injury and fibrogenesis caused by CCl4 in the rat model. Chronic liver injury may lead to development of fibrosis, a process in which HSC play a major role. As a result of liver injury, HSC, which in the healthy organ store vitamin A, undergo a process of activation that is mediated by the concerted action of resident hepatic cell types such as Kupffer cells, liver endothelial cells, and hepatocytes. The phenotype of activated HSC is characterized by α-smooth muscle actin (α-SMA) expression . α-SMA expression in the liver tissues is an indicator of hepatic stellate cell activation, which is recognized as being critical in liver fibrogenesis. Thus inhibition of the accumulation of activated HSCs is an important therapeutic strategy[34]. Our results showed the levels of α-SMA in rat liver tissues increased significantly after CCl4 administration for 12 wk. Emodin reduced α-SMA expression at mRNA and protein levels. Inflammation is commonly associated with hepatic fibrogenesis during chronic liver diseases[35]. CCl4 is metabolized in the liver by cytochrome P450 into the free radical CCl3[36]. The free radical attacks hepatocytes and causes necrosis of parenchymal cells, which promotes inflammatory responses in the liver[37]. Results in this study indicated that emodin suppressed inflammation caused by CCl4, which might lead to the protection of the liver from injury. It is now widely accepted that the pro-inflammatory cytokine TGF-β1 is a major cytokine in the regulation of the production, degradation, and accumulation of ECM[38], and it has been suggested that overexpression of TGF-β1 for a prolonged period of time after tissue damage may induce a fibroproliferative response and deposition of ECM, resulting in fibrosis in vital organs[39]. Many studies have detected the presence of TGF-β1, in the form of either protein or message, in the fibrotic tissues of animal models or human sam-ples[40]. Partial inhibition of the accumulation of ECM using either anti-TGF-β1 serum or a TGF-β1-binding protein has been reported in fibrosis models[41]. Our results showed that TGF-β1 mRNA levels and serum TGF-β1 protein levels in normal rat were low. After in-jection of CCl4 for 12 wk, mRNA and protein levels of TGF-β1 increased significantly. Emodin down-regulated mRNA levels of TGF-β1 expression in liver tissue. Furthermore, serum TGF-β1 levels in the model rats were also significantly down-regulated by emodin treatment in a manner similar to hepatic fibrosis attenuation. These findings imply that emodin might attenuate hepatic fibrosis through down-regulation of TGF-β1 expression in vivo. Smad4 is wel l known to function as one of the downstream effectors of TGF-β1, and it mediates TGF-β1-induced collagen synthesis[42] . Smads are intracellular signal transductive molecules of the TGF-β super family. According to differences in structure and function, nine Smads have been reported and classified into three groups. Smads 2 and 3 are named R-Smads in the pathway and Smad4 Co-Smads for all these pathways. Smads 6, 7, 8 are inhibitory factors of these Smads. When TGF-β1 binds to its receptor, Smad 2/3 is phosphorylated and binds with Smad4 and together they move into the nucleus for translation and expression of the target gene[43,44]. Smad signal transduction pathways are thought to play a crucial role in the process of liver damage and recovery, as well as liver fibrosis. These transcriptional responses appear to be mediated predominantly through Smad4. The widely held conclusion that Smad4 occupies a central role in transduction of TGF-β1 signals comes from multiple lines of biochemical and genetic evidence[45]. In reconstitution experiments, cell lines that lack Smad4 fail to respond to TGF-β1 signals, transfection of wildtype Smad4 restores the signaling capabilities of these cells[46]. Our study showed that both mRNA and protein expressions of Smad4 were remarkably up-regulated in fibrotic rats. We also observed down-regulation of Smad4 expression in emodin-treated fibrotic rats, suggesting that emodin attenuate hepatic fibrosis by regulating TGF-β1/smad signaling.In conclusion, the data presented herein provide evidence that emodin is active as an antifibrogenic drug able to reduce the biological effects of TGF-β1 in ongoing fibrogenesis. Giant Knotweed Rhizome, a traditional Chinese herbal medicine, is widely used in clinical practice for treating cirrhosis. Emodin, the main active monomer isolated from Giant Knotweed Rhizome, may be an attractive therapeutic agent for the treatment of fibrotic liver diseases. COMMENTS Background In the last decade, advances in the understanding of genes promoting hepatic stellate cell (HSC) activation are impressive. However, there are few breakthroughs in therapeutic intervention of hepatic fibrogenesis. Efficient and well-tolerated antifibrotic drugs are lacking and current treatment of hepatic fibrosis is limited to withdrawal of the noxious agent. Research identifying innocuous antifibrotic agents is of high priority and urgently needed. Research frontiers Emodin is efficacious in the management of hepatic fibrosis. However, the mechanisms underlying its effects remain to be elucidated. The current study evaluates the in vivo role of emodin in the protection of the liver from fibrogenesis caused by carbon tetrachloride (CCl4) in a rat model and further explores the underlying mechanisms. Innovations and breakthroughs To the best of the authors’ knowledge, this is the first study to report that emodin protects the liver from CCl4-induced fibrogenesis by inhibiting activation of HSC via modulating transforming growth factor-β1 (TGF-β1)/Smad signaling pathways. Results in this study provide novel insight into the mechanisms of emodin in the protection of the liver. Applications By evaluating the role of emodin in protecting the liver against fibrogenesis caused by carbon tetrachloride (CCl4) in rats via inhibition of hepatic stellate cells activation, emodin might be a therapeutic antifibrotic agent for the treatment of hepatic fibrosis. Terminology Smad4 is a protein which in humans is encoded by the SMAD4 gene. SMAD4 is a 552 amino acid protein involved in cell signaling. It is the only known mammalian coSmad. It is a homolog of the Drosophila protein: “Mothers against decapentaplegic”. Peer review This study examines the effects of emodin on CCl4-induced liver fibrosis. The authors show reduced fibrosis, decreased stellate cell smooth muscle actin expression and decreased TGF-β expression. The study has been suitably designed and clearly reported. REFERENCES 1 Farci P, Roskams T, Chessa L, Peddis G, Mazzoleni AP, Scioscia R, Serra G, Lai ME, Loy M, Caruso L, Desmet V, Purcell RH, Balestrieri A. Long-term benefit of interferon alpha therapy of chronic hepatitis D: regression of advanced hepatic fibrosis. Gastroenterology 2004; 126: 1740-1749 2 Satapathy SK, Sakhuja P, Malhotra V, Sharma BC, Sarin SK. Beneficial effects of pentoxifylline on hepatic steatosis, fibrosis and necroinflammation in patients with non-alcoholic steatohepatitis. J Gastroenterol Hepatol 2007; 22: 634-638 3 Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med 2007; 13: 1324-1332 4 Decologne N, Kolb M, Margetts PJ, Menetrier F, Artur Y, Garrido C, Gauldie J, Camus P, Bonniaud P. TGF-beta1 induces progressive pleural scarring and subpleural fibrosis. J Immunol 2007; 179: 6043-6051 5 Yao Q, Pawlaczyk K, Ayala ER, Styszynski A, Breborowicz A, Heimburger O, Qian JQ, Stenvinkel P, Lindholm B, Axelsson J. The role of the TGF/Smad signaling pathway in peritoneal fibrosis induced by peritoneal dialysis solutions. Nephron Exp Nephrol 2008; 109: e71-e78 6 Yang Y, Yang S, Chen M, Zhang X, Zou Y, Zhang X. Compound Astragalus and Salvia miltiorrhiza Extract exerts anti-fibrosis by mediating TGF-beta/Smad signaling in myofibroblasts. J Ethnopharmacol 2008; 118: 264-270 7 De Minicis S, Seki E, Uchinami H, Kluwe J, Zhang Y, Brenner DA, Schwabe RF. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology 2007; 132: 1937-1946 8 Cheng K, Mahato RI. Gene modulation for treating liver fibrosis. Crit Rev Ther Drug Carrier Syst 2007; 24: 93-1469 Yang YC, Lim MY, Lee HS. Emodin isolated from Cassia obtusifolia (Leguminosae) seed shows larvicidal activity against three mosquito species. J Agric Food Chem 2003; 51: 7629-7631 10 Yim H, Lee YH, Lee CH, Lee SK. Emodin, an anthraquinone derivative isolated from the rhizomes of Rheum palmatum, selectively inhibits the activity of casein kinase II as a competitive inhibitor. Planta Med 1999; 65: 9-13 11 Guo JM, Xiao BX, Liu Q, Zhang S, Liu DH, Gong ZH. Anticancer effect of aloe-emodin on cervical cancer cells involves G2/M arrest and induction of differentiation. Acta Pharmacol Sin 2007; 28: 1991-1995 12 Shuangsuo D, Zhengguo Z, Yunru C, Xin Z, Baofeng W, Lichao Y, Yanan C. Inhibition of the replication of hepatitis B virus in vitro by emodin. Med Sci Monit 2006; 12: BR302-BR306 13 Chang CH, Lin CC, Yang JJ, Namba T, Hattori M. Anti-inflammatory effects of emodin from ventilago leiocarpa. Am J Chin Med 1996; 24: 139-142 14 Zhan Y, Li D, Wei H, Wang Z, Huang X, Xu Q, Lu H. Emodin on hepatic fibrosis in rats. Chin Med J (Engl) 2000; 113: 599-601 15 Zhan YT, Liu B, Li DG, Bi CS. [Mechanism of emodin for anti-fibrosis of liver] Zhonghua Ganzangbing Zazhi 2004; 12: 245-246 16 Proctor E, Chatamra K. High yield micronodular cirrhosis in the rat. Gastroenterology 1982; 83: 1183-119017 Abe W, Ikej ima K, Lang T, Okumura K, Enomoto N, Kitamura T, Takei Y, Sato N. Low molecular weight heparin prevents hepatic fibrogenesis caused by carbon tetrachloride in the rat. J Hepatol 2007; 46: 286-294 18 [Guidelines for diagnosis and treatment of nonalcoholic fatty liver diseases] Zhonghua Ganzangbing Zazhi 2006; 14: 161-163 19 Nouchi T, Worner TM, Sato S, Lieber CS. Serum procollagen type III N-terminal peptides and laminin P1 peptide in alcoholic liver disease. Alcohol Clin Exp Res 1987; 11: 287-291 20 Kim KM, Choi WB, Park SH, Yu E, Lee SG, Lim YS, Lee HC, Chung YH, Lee YS, Suh DJ. Diagnosis of hepatic steatosis and fibrosis by transient elastography in asymptomatic healthy individuals: a prospective study of living related potential liver donors. J Gastroenterol 2007; 42: 382-388 21 Livak KJ, Schmi t tgen TD. Analysis of relat ive gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402-408 22 Bialek SR, Redd JT, Lynch A, Vogt T, Lewis S, Wilson C, Bell BP. Chronic liver disease among two American Indian patient populations in the southwestern United States, 2000-2003. J Clin Gastroenterol 2008; 42: 949-954 23 Adrian JE, Poelstra K, Scherphof GL, Meijer DK, van Loenen-Weemaes AM, Reker-Smit C, Morselt HW, Zwiers P, Kamps JA. Effects of a new bioactive lipid-based drug carrier on cultured hepatic stellate cells and liver fibrosis in bile duct-ligated rats. J Pharmacol Exp Ther 2007; 321: 536-543 24 Prudhomme GJ. Pathobiology of transforming growth factor beta in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab Invest 2007; 87: 1077-1091 25 Song M, Song Z, Barve S, Zhang J, Chen T, Liu M, Arteel GE, Brewer GJ, McClain CJ. Tetrathiomolybdate protects against bile duct ligation-induced cholestatic liver injury and fibrosis. J Pharmacol Exp Ther 2008; 325: 409-416 26 Pihusch V, Pihusch M, Penovici M, Kolb HJ, Hiller E, Pihusch R. Transforming growth factor beta-1 released from platelets contributes to hypercoagulability in veno-occlusive disease following hematopoetic stem cell transplantation. Thromb Res 2005; 116: 233-240 27 Zou YH, Yang Y, Li J, Wu Q, Li WP, Lu JT, Roberts MS. Potential therapeutic effects of a traditional Chinese formulation, BJ-JN, on liver fibrosis induced by carbon tetrachloride in rats. J Ethnopharmacol 2008; 120: 452-457 28 Yuan LP, Chen FH, Ling L, Bo H, Chen ZW, Li F, Zhong MM, Xia LJ. Protective effects of total flavonoids of Bidens bipinnata L. against carbon tetrachloride-induced liver fibrosis in rats. J Pharm Pharmacol 2008; 60: 1393-1402 29 You S, Zhou M, Xue B, Fang T, Jiang W, Li C, Xu H, Jiang J, Wang Y, Xu S. A clinical study on bing gan ling oral liquid for treatment of hepatitis C. J Tradit Chin Med 1998; 18: 209-214 30 Weiler-Normann C, Herkel J, Lohse AW. Mouse models of liver fibrosis. Z Gastroenterol 2007; 45: 43-50 31 Smyth R, Munday MR, York MJ, Clarke CJ, Dare T, Turton JA. Comprehensive characterization of serum clinical chemistry parameters and the identification of urinary superoxide dismutase in a carbon tetrachloride-induced model of hepatic fibrosis in the female Hanover Wistar rat. Int J Exp Pathol 2007; 88: 361-376 32 Petlevski R, Hadzija M, Bajalo JL, Juretić D. Effect of acarbose on alanine aminotransferase and aspartate aminotransferase activities in the liver of control and diabetic CBA mice. Acta Pharm 2006; 56: 87-93 33 Tajima K, Terai S, Takami T, Kawaguchi K, Okita K, Sakaida I. Importance of inhibitor of DNA binding/differentiation 2 in hepatic stellate cell differentiation and proliferation. Hepatol Res 2007; 37: 647-655 34 Tu CT, Guo JS, Wang M, Wang JY. Antifibrotic activity of rofecoxib in vivo is associated with reduced portal hypertension in rats with carbon tetrachloride-induced liver injury. J Gastroenterol Hepatol 2007; 22: 877-884 35 Luedde T, Trautwein C. A molecular l ink between inflammation and fibrogenesis: the bacterial microflora influences hepatic fibrosis via toll-like receptor 4-dependent modification of transforming growth factor-beta signaling in hepatic stellate cells. Hepatology 2008; 47: 1089-1091 36 Mochizuki M, Shimizu S, Urasoko Y, Umeshita K, Kamata T, Kitazawa T, Nakamura D, Nishihata Y, Ohishi T, Edamoto H. Carbon tetrachloride-induced hepatotoxicity in pregnant and lactating rats. J Toxicol Sci 2009; 34: 175-181 37 Chou WY, Lu CN, Lee TH, Wu CL, Hung KS, Concejero AM, Jawan B, Wang CH. Electroporative interleukin-10 gene transfer ameliorates carbon tetrachloride-induced murine liver fibrosis by MMP and TIMP modulation. Acta Pharmacol Sin 2006; 27: 469-476 38 Kottler UB, Jünemann AG, Aigner T, Zenkel M, Rummelt C, Schlötzer-Schrehardt U. Comparative effects of TGF-beta 1 and TGF-beta 2 on extracellular matrix production, proliferation, migration, and collagen contraction of human Tenons capsule fibroblasts in pseudoexfoliation and primary open-angle glaucoma. Exp Eye Res 2005; 80: 121-134 39 Xu Q, Norman JT, Shrivastav S, Lucio-Cazana J, Kopp JB. In vitro models of TGF-beta-induced fibrosis suitable for high-throughput screening of antifibrotic agents. Am J Physiol Renal Physiol 2007; 293: F631-F640 |







 Emodin protects rat liver from CCl4-induced fibrogenesis via inhibition of hepatic stellate cells activation
Emodin protects rat liver from CCl4-induced fibrogenesis via inhibition of hepatic stellate cells activation