| The role of chlorogenic acid in the resistance of apples to apple scab |
| 发布时间:2012-07-19 信息来源:admin 发布人:admin 点击次数:3514 |
The role of chlorogenic acid in the resistance of apples to apple scab (Venturia inaequalis (Cooke) G. Wind. Aderh.)a Abstract The content of chlorogenic acid was studied in three scab resistant apple cultivars (‘Topaz’, ‘Gold Rush’, ‘Goldstar’) and two scab susceptible ones (‘Golden Delicious Weinsberg’, ‘Golden Delicious Clone B’) growing in Laboratory Field of the Biotechnical Faculty in Ljubljana. The leaf samples were collected four times in 30 day intervals between 12 June and 10 September 2001 and analysed using the system of high pressure liquid chromatography (HPLC). The content of chlorogenic acid was determined by means of a standard. It did not differ statistically significantly between the resistant cultivars and the two susceptible ones. At all four dates, leaves of the cultivar ‘Goldrush’ contained statistically significantly larger quantities of chlorogenic acid than leaves of the remaining scab resistant and scab susceptible cultivars. The content of chlorogenic acid changed during the growing period: it was the highest in July or August while in September it declined. The scab infection of the leaves of scab susceptible cultivars affected the accumulation of chlorogenic acid in the way that its content increased with the degree of infection. Greater amount of chlorogenic acid was contained in the leaves of the cultivar ‘Golden Delicious Weinsberg’ than in the leaves of the cultivar ‘Golden Delicious Clone B’.
Key words: fruit growing, apple, Malus domestica, cultivars, resistance, phenolic compounds, chlorogenic acid, leaves, apple scab, Venturia inaequalis, growing season
1 Introduction
Apple scab has been and still is the most serious disease occurring in the commercial apple orchards (Treutter and Feucht, 1990), and it is for this reason that breeders concentrated on developing scab immunity before targeting any other disease. To keep the pathogens away after wounding, plants have developed various biochemical defence mechanisms. Rapid synthesis of phenolic derivatives which are involved in disease resistance is a well known response (Schwalb and Feucht, 1999). Plants produce naturally more than 8000 different phenolic compounds for functions as varied as cell wall biosynthesis, host defence and their contribution to the colour, taste and flavour characteristics of fruits. Phenolic compounds are also used as indicators of the physiological stages during the fruit development (Macheix et al., 1990).
Polyphenols are located in the cell vacuoles (Treutter et al., 1986). Since the first suggestions that phenolic intermediates have a role in the active expression of resistance, an underlying problem in ascertaining that such secondary metabolites are of primary importance has been the localization and timing of the host response (Nicholson, 1992). The phenolic derivatives can oxidize and react with proteins, thus causing a loss of enzyme function, and restricting the viability of aggressors or they can be deposited inside the cell wall as an important first line in plant defence against infection (Schwalb and Feucht, 1999).
A plant can respond to fungal infection by the synthesis of antifungal agent at the site of attack. The so called phytoalexins (Strack, 1997) are toxic antimicrobial substances produced in appreciable amounts in plants only after the stimulation by various types of phytopathogenic micro-organisms or by chemical and mechanical injury. The production and accumulation of phytoalexins occurs in healthy plant cells surrounding wounded or infected cells, and they are stimulated by alarm substances produced and released by the damaged cells and diffusing into the adjacent healthy cells. The most known phytoalexins are toxic, too, and inhibit the growth of fungi pathogenic to plant (Agrios, 1997).
Many of the phytoalexins subsequently characterised from a variety of plant sources belong to the phenolic class. Phytoalexins synthesized and accumulated in plants after the exposure to signals from micro-organism (Kindl, 1994). The patterns of their distribution will vary between different organs and within different populations of the same plant species. The extent of the phytoalexin response is highly dependent on the MIKULIČ PETKOVŠEK, M., USENIK, V., ŠTAMPAR, F.: The role of chlorogenic acid... tissue being stressed (Harborne, 1994). Chemical stress, mechanical or biological stress changes the phenolic metabolism of apples. These changes on the phenolic level can play a role in protection of the plant (Mayr et al., 1994).
The resistance genes of resistant apple genotypes possibly act as regulatory genes of phenol synthesis (Michalek et al., 1999). Phenols are always present in conjugated form, usually with glycosidic attachment. They may be released in the free form during the fungal infection or insect grazing through enzymatic or other hydrolysis. In such cases they are likely to be much more toxic to the invading organism than the bound form. Any free diphenols liberated during fungal infection may well undergo oxidation to the related quinones, of which the toxicity is greater than that of the original phenol (Harborne, 1994).
The activity of many phenol-oxidizing enzymes is generally higher in the infected tissue of the resistant cultivars than in the infected tissue of the susceptible ones or in the uninfected tissue of the healthy plants (Agrios, 1997). Singler and Stösser (1990) did not find a close correlation between the occurrence and/or distribution of phenolic substances in the epi- or hypodermal fruit cells and scab susceptibility of different cultivars.
Simple esters of hydroxycinnamic acids with quinic acid are termed chlorogenic acid (Macrae et al., 1993). Chlorogenic acid is the most important cinnamic acid derivative in fruits (Van Buren, 1970) and is known as antioxidant and radical scavenger, and has also been shown to inhibit lipoxygenase activity in leaves (Torel and Cillard, 1986). It had demonstrable antifungal properties when tested in vitro against pathogenic fungi (Harborne, 1994). Rat –Morris et al. (1996) established that apple leaves resistant to Venturia inaequalis have a significantly higher concentration of chlorogenic acid than the susceptible ones.
Measurements of the content of chlorogenic acid during growing season showed that the content was the highest early in the season and decreased as the fruit matured (Ndubizu, 1976; Mayr et al. 1995). Mayr et al. (1994) established that the concentrations of chlorogenic acid declined during fruit development from June to September continuously.
We studied the polyphenolic pattern in apple leaves to find a relation between the levels of some phenolic compounds present in leaves and scab resistance that could be useful to screen resistant and susceptible apple cultivars. The hypothesis that resistant varieties have the ability (under genetic control) to induce a specific metabolic environment leading to fungus inhibitors could be considered.
2 Material and method
In the research project, phenolic compounds in the leaves of three scab resistant apple cultivars (‘Topaz’, ‘Goldstar’, ‘Gold Rush’) and two scab susceptible ones (‘Golden Delicious Weinsberg’, ‘Golden Delicious Clone B’) were studied. In the period of 12 June to 10 September 2001, apple leaves were picked four times at 30 day intervals in the orchard belonging to Laboratory Field of the Biotechnical Faculty in Ljubljana. The leaves of the cultivar ‘Golden Delicious Clone B’ were collected in a standard orchard (14-year-old trees on the rootstock M 9), and the leaves of the remaining cultivars in a biological one (3-year-old Zb. Bioteh. Fak. Univ. Ljublj. Kmet. 81 - 2,oktober 2003
trees on the rootstock M 9). Phenolic compounds were extracted from the leaves. The samples were then analysed using the system of high pressure liquid chromatography (HPLC), and the data were processed statistically.
For the analyses of polyphenol samples were extracted with acetone-water (80:20, v/v) containing Triton X-100 (0.4%) for 10 days at 4°C according to Treutter (1988). In a mortar, 100 mg of plant material was homogenized with 20 ml of extraction solution. The mixture was stored in a refrigerator at 4°C in closed plastic containers (made of PEP – polyethylenepropylene). It was left tightly closed in the refrigerator during the entire extraction. After the extraction the samples were decanted into small glasses. The solvent was evaporated at 40°C. The residue was dissolved in methanol (2 ml). The samples were decanted in centrifuge tubes and waited until the content was deposited. Before injecting the samples onto the column they were filtered through membranes (Sartorious RC 25, 0.45 µm pore size). The samples were stored at -20°C until they were submitted to HPLC analysis.
Polyphenols were determined with reversed phase HPLC of Thermo Separation Products (TSP). The HPLC equipment consisted of X-ACTTM degasser, P2000 TSP pump, Chromsep SS (250 x 4.6 mm) Hypersil 5 ODS, reversed phase column, guard column Chromsep Guard SS (10 x 3 mm) reversed phase, autosampler AS 1000, detector WellChrom K-2500 for detection at 280 nm (UV), and OS/2 Warp IBM Operating System (1994). Solvent A was 5% formic acid and solvent B methanol with a gradient range according to Treutter et al. (1994). The gradient range was: 0-5 min, isocratic, 5% B in A; 5-15 min, 5-10% B in A; 15-30 min, isocratic, 10% B in A; 30-50 min, 10-15% B in A, 50-70 min, isocratic, 15% B in A; 70-85 min, 15-20% B in A; 85-95 min, isocratic, 20% B in A, 95-110 min, 20-25% B in A, 110-140 min, 25-30% B in A; 140-160 min, 30-40% B in A; 160-175 min, 40-50% B in A; 175-190 min, 50-90% B in A. The flow rate was 0.5 ml/min. The injection volume was 5 µl.
The individual phenolic compounds were identified by comparison of their UV absorbance spectra with authentic samples (Fluka Chemical).
Statistical analysis was done with the program Statgraphics Plus 4.0. The differences between treatments (n>3) were tested with Duncan test. A risk of 5% was taken into consideration.
3 Results and discussion
3.1 Comparison of the content of chlorogenic acid between resistant and susceptible cultivars
The comparison of the content of chlorogenic acid in healthy leaves of different apple cultivars showed a statistically significant difference between the content of chlorogenic acid in the leaves of the cultivar ‘Gold Rush’ and other cultivars. In June, the content of chlorogenic acid in the leaves of the cultivar ‘Gold Rush’ was almost three times as high as in the remaining cultivars. In July, the difference was even greater. In August, the content of chlorogenic acid increased statistically insignificantly in all cultivars. In September, the content of chlorogenic acid in the leaves of the cultivar ‘Topaz’ decreased and it remained unchanged in the leaves of the cultivars ‘Goldstar’, ‘Golden Delicious Weinsberg’ and ‘Golden Delicious Clone B’ while in the leaves of the cultivar ‘Gold Rush’ it increased to 1.03 mg/g of fresh weight of a leaf (Table 1). In other two scab resistant cultivars, ‘Topaz’ and Goldstar’, the content of chlorogenic acid did not differ significantly from the content in the leaves of two scab susceptible cultivars, ‘Golden Delicious Weinsberg’ and ‘Golden Delicious Clone B’, with the exception of the months July and August MIKULIČ PETKOVŠEK, M., USENIK, V., ŠTAMPAR, F.: The role of chlorogenic acid...
when the leaves of the cultivar ‘Goldstar’ contained significantly more chlorogenic acid in comparison with the cultivar ‘Golden Delicious Clone B’.
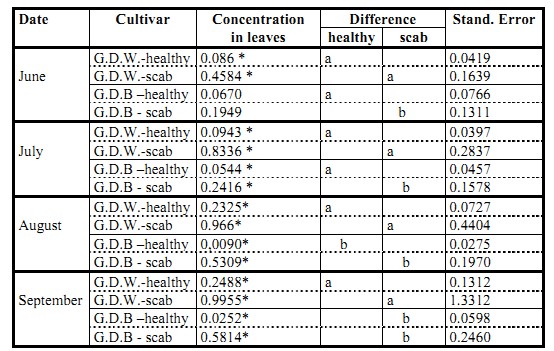
Table 1: The content of chlorogenic acid (mg/g) in healthy leaves of different apple cultivars
- average contents of phenolic compounds in columns designated with the same letter do not differ statistically significantly between them (Duncan’s test, p < 0.05). Average contents of phenolic compounds were compared between individual apple cultivars for each date separately
The results agree partly with the findings by Rat-Morrise et al. (1996) which reported twice as much chlorogenic acid in the leaves of scab resistant apple cultivars than in the leaves of scab susceptible cultivars. Rat-Morrise et al. (1996) and, prior to them, Williams and Kuč (1969), linked the quantitative differences in the content of chlorogenic acid with the resistance of apples to scab.
3.2 Changes in the content of chlorogenic acid during the growing period
In the research we also studied how the content of chlorogenic acid changed during the growing period. The average content of chlorogenic acid in the leaves of the cultivar ‘Topaz’ was the lowest in June; it was increasing slowly up to August to decrease slowly by September. There were no statistically significant differences in the content of chlorogenic acid in the leaves of the cultivar ‘Topaz’ between the dates discussed. Zb. Bioteh. Fak. Univ. Ljublj. Kmet. 81 - 2, oktober 2003 The cultivar ‘Gold Rush’ also presented the lowest content of chlorogenic acid in June. In July, the content of chlorogenic acid in leaves increased statistically significantly. The average content increased a little more in August but there were no statistical differences in the content if compared to July. In September, the content of chlorogenic acid in the leaves of the cultivar ‘Gold Rush’ increased statistically significantly again.
In June, the content of chlorogenic acid was the lowest in the leaves of the cultivar ‘Goldstar’. In July, the average content increased but there were no statistically significant differences between July and June. In August, the average content of chlorogenic acid increased a bit so that statistically significant differences appeared if compared to June while there were no statistically significant differences between July and August. In September, the average content of chlorogenic acid in the leaves of the cultivar ‘Goldstar’ decreased; however, the decrease was not statistically significant.
The average content of chlorogenic acid in the leaves of the cultivar ‘Golden Delicious Weinsberg’ was the lowest in July, but the difference was not statistically significant if compared to June. In August, the average content increased as well as in September. Statistically significant differences in the content of chlorogenic acid were observed between the contents in July – the highest - and in September – the lowest.
In the cultivar ‘Golden Delicious Clone B’ the average content of chlorogenic acid in leaves declined from June until August and augmented in September if compared to August. There were no statistically significant differences in the content of chlorogenic acid in the leaves of the cultivar ‘Golden Delicious Clone B’ between individual dates.
Figure 1: The content of chlorogenic acid (mg/g) in the healthy leaves of individual apple cultivars measured at different dates. Aug SepMIKULIČ PETKOVŠEK, M., USENIK, V., ŠTAMPAR, F.: The role of chlorogenic acid... 3.3 The change of the content of chlorogenic acid due to infection with apple scab
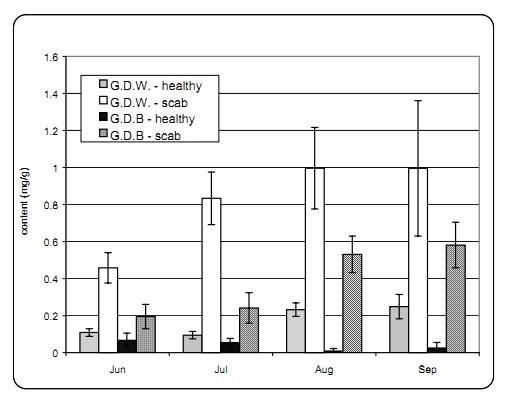
The comparison of the content of chlorogenic acid between healthy and scab infected leaves of the cultivar ‘Golden Delicious Weinsberg’ indicated statistically significant differences at all four dates. There was statistically significantly less chlorogenic acid in healthy leaves than in infected ones. The content of chlorogenic acid has increased both in healthy and in infected leaves from June to September (Table 2).
In healthy leaves of the cultivar ‘Golden Delicious Clone B’ there was statistically significantly less chlorogenic acid than in infected leaves at all four dates. The content of chlorogenic acid was increasing in healthy and infected leaves during the growth time.
Table 2: The content of chlorogenic acid (mg/g) in healthy leaves and in those infected with apple scab of the cultivars ‘Golden Delicious Weinsberg’ (G.D.W.) and ‘Golden Delicious Clone B’ (G.D.B) determined at different dates.
* - average contents of phenolic compounds designated with “*” do not differ statistically significantly (Duncan’s test, p < 0,05). Comparison was made between healthy and infected leaves of the same apple cultivar at the same date. a - average contents of phenolic compounds in columns designated with the same letter do not differ statistically significantly between them (Duncan’s test, p < 0,05). Average contents of phenolic compounds were compared between healthy leaves of two apple cultivars (‘Golden Delicious Weinsberg’ and ‘Golden Delicious Clone B’). The second comparison was made between infected leaves.
The comparison between healthy leaves of the cultivars ‘Golden Delicious Weinsberg’ and ‘Golden Delicious Clone B’ indicated that the average content of chlorogenic acid was higher in the leaves of the cultivar ‘Golden Delicious Zb. Bioteh. Fak. Univ. Ljublj. Kmet. 81 - 2, oktober 2003 Weinsberg’ in the months August and September. In June and July, the differences were not statistically significant. Similar applies to the comparison of infected leaves of both cultivars, i.e. the infected leaves of the cultivar ‘Golden Delicious Weinsberg’ contained statistically significantly more chlorogenic acid than the infected leaves of the cultivar ‘Golden Delicious Clone B’ at all dates. The reason for that may be attributed to a better condition of the trees of cultivar ‘Golden Delicious Clone B’ and/or to a longer exposure of the trees of ‘Golden Delicious Weinsberg’ to various stress factors.
The results obtained in previous investigations indicate that apple respond to fungus infection by accumulating phenols. Michalek et al. (1996) reported about the predominant accumulation of derivatives of caffeic acid while Bennett and Wallsgrove (1994) found that in some cases, after the infection of apples with the fungus Venturia inaequalis, it comes to an accumulation of the derivatives of chlorogenic and coumaric acid. The content of chlorogenic acid can also be influenced by other factors which impose a mechanical stress to the plant. Mayr et al. (1994) established that it was impossible to find out how much was the content of phenolic compounds in a plant influenced by a particular stress factor since it is the result of mutual effects of all stress and other environmental factors.
Figure 2: The content of chlorogenic acid (mg/g) in healthy leaves and in those infected with apple scab of the cultivars ‘Golden Delicious Weinsberg’ (G.D.W.) and ‘Golden Delicious Clone B’ (G.D.B) determined at different dates MIKULIČ PETKOVŠEK, M., USENIK, V., ŠTAMPAR, F.: The role of chlorogenic acid...
4 Conclusion
The results of our experiment in which we compared the content of chlorogenic acid in the leaves of three scab resistant apple cultivars and two scab susceptible ones did not show any differences in the content of this phenolic compound between the two apple cultivar types. The content of chlorogenic acid was statistically significantly high only in the leaves of the cultivar ‘Gold Rush’ on all four dates. In the remaining two scab resistant cultivars, ‘Topaz’ and ‘Goldstar’, the content of chlorogenic acid did not differ significantly from that in the leaves of scab susceptible cultivars ‘Golden Delicious Weinsberg’ and ‘Golden Delicious Clone B’. Significant difference was observed only between the cultivar ‘Goldstar’ and ‘Golden Delicious Clone B’ in July and August.
In the resistant cultivars the content of chlorogenic acid in leaves increased from June to August but the changes were not substantial. In September, the content of chlorogenic acid in the leaves of the cultivar ‘Gold Rush’ increased while in the cultivars ‘Topaz’ and ‘Goldstar’ it decreased. In the susceptible cultivar ‘Golden Delicious Weinsberg’ the content of chlorogenic acid in August leaves increased if compared to the content in July leaves while in the cultivar ‘Golden Delicious Clone B’ the content of chlorogenic acid in August decreased if compared with the July content. There were no significant differences between the June and July, and August and September contents of chlorogenic acid in the leaves of both susceptible cultivars. In both scab susceptible cultivars, ‘Golden Delicious Weinsberg’ and ‘Golden Delicious Clone B’, essentially higher content of chlorogenic acid was found in infected than in healthy leaves at all four dates. The comparison between the cultivars of healthy and infected leaves was an interesting issue. Both healthy and infected leaves of the cultivar ‘Golden Delicious Weinsberg’ contained more chlorogenic acid than healthy and infected leaves of the cultivar ‘Golden Delicious Clone B’,
5 References
Agrios N.G. 1997. Plant Pathology. 4th edition. San Diego, Academic Press San Diego: 635 str. Bennett R.N., Wallsgrove R.M. 1994. Secondary metabolites in plant defence mechanisms. New Phytologist, 127: 617 – 633. Harborne J.B., 1994. Do natural plant phenols play a role in ecology? Acta Horticulturae, 381: 36-43. Kindl H. 1994. Biochemical mechanism controlling the formation of phenols in plants. Acta Horticulturae, 381: 176-184. Macheix J.J., Fleuriet A., Billot J. 1990. Fruit phenolics. Boca Raton, CRC Press: 292 str. Macrae R., Robinson R. K., Sadler M. J. 1993. Phenolic compounds. In: Encyclopedia of food science, food technology and nutrition. London, Academic press, 3548-3553. Mayr. U., Batzdorfer R., Treutter D., Feucht W. 1994. Surfactant-induced changes in phenol content of apple leaves and fruit skins. Acta Horticulturae, 381: 479-487. Mayr U., Treutter D., Santos-Buelga C., Bauer H., Feucht W. 1995. Developmental changes in the phenol concentrations of Golden delicious apple fruits and leaves. Phytochemistry, 35, 5: 1151-1155. Zb. Bioteh. Fak. Univ. Ljublj. Kmet. 81 - 2, oktober 2003
242 Michalek S., Treutter D., Mayr U., Lux-Endrich A., Gutmann., Feucht W. 1996. Role of flavan-3-ols in resistance of apple trees to Venturia inaequalis. In: Polyphenols Communications 96. 18th international conference on polyphenols, Bordeaux, 15- 18 jul. 1996. Vercauteren J., Cheze C., Dumon M., Weber J. (eds.). Bordeaux, Secretariat du Groupe Polyphenols: 26-27. Michalek S., Mayr U., Treutter D., Lux-Endrich A., Gutmann M., Feucht W., Geibel M. 1999. Role of flavan-3-ols in resistance of apple trees to Venturia inaequalis. Acta Horticulturae, 484: 535-539. Ndubizu T.O.C. 1976. Relations of phenolic inhibitors to resistance of immature apple fruits to rot. Journal of Horticultural Science, 51: 311-319. Nicholson R.L. 1992. Phenolic compounds and their role in disease resistance. Annual Review of Phytopathology, 30: 369-389. Rat-Morris E., Amiot M.J., Tacchini M., Lespinasse Y. 1996. Phenolic composition of apple leaves, prior to infestation, associated with the resistance of the apple cv. Florina- Querina to the apple scab Venturia inaequalis and to the Rosy apple aphid Dysaphis plantaginea. In: Polyphenols Communications 96. 18th international conference on polyphenols, Bordeaux, 15-18 jul. 1996. Vercauteren J., Cheze C., Dumon M., Weber J. (eds.) Bordeaux, Secretariat du Groupe Polyphenols: 355- 356. Schwalb P., Feucht W. 1999. Changes in the concentration of phenolic substances in the bark during the annual development of the cherry tree (Prunus avium L.). Advances in Horticultural Science, 13: 71-75. Singler D., Stösser R. 1990. Der Apfelschorf (Venturia inaequalis): Histologische Untersuchungen an Früchten von resistenten und anfälligen Sorten. Mitteilungen Klosterneuburg, 42: 223-229. Strack D. 1997. Plant biochemistry. In: Phenolic metabolism. Dey P.M., Harborne J.B. (eds). London, Academic Press: 387-416. Torel J., Cillard P. 1986. Antioxidant activity of flavonoids and reactivity with peroxy radical. Phytochemistry, 25: 382-386. Treutter D., Feucht W., Schmid P.P.S. 1986. Polyphenole des Phloems in Beziehung zur Inkompatibilität von interspezifischen Prunus-Veredlungen (Prunus avium L., Prunus cerasus L.). Flavanone und Flavanole über der Veredlungsstelle. Gartenbauwissenschaft, 51: 77-84. Treutter D. 1988. Separation of naturally occurring mixtures of phenolic compounds from various Prunus tissues by reversed-phase high-performance liqud chromatography. Journal of Chromatography, 436: 490-496. Treutter D., Feucht W. 1990. The pattern of flavan-3-ols in relation to scab resistance of apple cultivars. Journal of Horticultural science, 65, 5: 511-517. Treutter D., Santos-Buelga C., Gutmann M., Kolodziej H. 1994. Identification of flavan-3-ols and procyanidins by high-performance chromatography and chemical reaction detection. Journal of Chromatography A, 667: 290-297. Van Buren J. 1970. Fruit phenolics. In: The biochemistry of fruits and their products. Vol. 1. Hulme A. C. (ed.). New York, Academic Press: 269-304. Williams E.B., Kuć J. 1969. Resistance in Malus to Venturia inaequalis. Annual Review of Phytopathology, 2: 223-246. |







 The role of chlorogenic acid in the resistance of apples to apple scab
The role of chlorogenic acid in the resistance of apples to apple scab