| Chlorogenic Acids from Green Coffee Extract are Highly Bioavailable in Humans1,2 |
| 发布时间:2012-07-19 信息来源:admin 发布人:admin 点击次数:5335 |
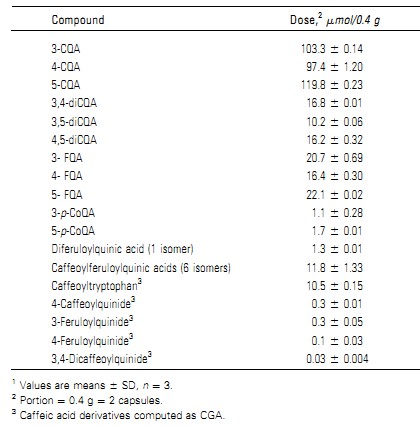
Chlorogenic Acids from Green Coffee Extract are Highly Bioavailable in Humans1,2 Abstract Chlorogenic acids (CGA) are cinnamic acid derivatives with biological effects mostly related to their antioxidant and antiinflammatory activities. Caffeoylquinic acids (CQA) and dicaffeoylquinic acids (diCQA) are the main CGA found in nature.Because green coffee is a major source of CGA, it has been used for production of nutraceuticals. However, data on the bioavailability of CGA from green coffee in humans are inexistent. The present study evaluated the pharmacokinetic profile and apparent bioavailability of CGA in plasma and urine of 10 healthy adults for 8 h after the consumption of a decaffeinated green coffee extract containing 170 mg of CGA. Three CQA, 3 diCQA, and caffeic, ferulic, isoferulic, and p-coumaric acids were identified in plasma by HPLC-Diode Array Detector-MS after treatment. Over 30% (33.1 6 23.1%) of the ingested cinnamic acid moieties were recovered in plasma, including metabolites, with peak levels from 0.5 to 8 h after treatment.CGA and metabolites identified in urine after treatment were 4-CQA, 5-CQA, and sinapic, p-hydroxybenzoic, gallic, vanillic, dihydrocaffeic, caffeic, ferulic, isoferulic, and p-coumaric acids, totaling 5.5 6 10.6% urinary recovery of the ingested cinnamic and quinic acid moiteties. This study shows that the major CGA compounds present in green coffee are highly absorbed and metabolized in humans. J. Nutr. 138: 2309–2315, 2008. Introduction Chlorogenic acids (CGA)5 are phenolic compounds formed by the esterification of cinnamic acids, such as caffeic, ferulic, and p-coumaric acids, with (-)-quinic acid. A series of health benefits have been associatedwith the consumption of CGA in the last few years, such as reduction of the relative risk of cardiovascular disease, diabetes type 2, and Alzheimer’s disease (1–3), and antibacterial and antiinflammatory activities (4,5). Their lactones also have been shown to exert positive effects in rats such as enhancement of insulin action (6). Green (or raw) coffee is a major source of CGA in nature (5–12 g/100 g) (7). Recent studies demonstrated that the consump- tion of green coffee extracts produced antihypertensive effect in rats and humans (8,9), improvement in human vasoreactivity(10), inhibitory effect on fat accumulation and body weight in mice and humans (11,12), and modulation of glucose metab-olism in humans (13). Such biological effects have been attributed to CGA present in green coffee. The major CGA in green coffee are 3-, 4-, and 5-caffeoylquinic acids (3-, 4-, and 5-CQA),3,4-, 3,5-, and 4,5-dicaffeoylquinic acids (3,4-, 3,5-, and 4,5-diCQA),3-, 4-, and 5-feruloylquinic acids (3-, 4-, and 5-FQA), and 3-, 4-,and 5-p-coumaroylqunic acids (3-, 4-, and 5-p-CoQA). Caf-feoylferuloylquinic acids are minor CGA compounds also found in green coffee, especially in C. canephora species. Very small amounts of CGA lactones formed by heating during primary processing may be also observed (7,14). Even though green coffee has been used for production of nutraceuticals (12), data on the pharmacokinetic profile and bioavailability of CGA from this food matrix are still inexistent. Moreover, although we have recently reported the pharmacokinetic profile of the main CGA compounds in humans after roasted coffee consumption (15), the bioavailability of CGA compounds from a food matrix has not been reported in animals or humans so far. Therefore, the objective of this study was to evaluate the pharmacokinetic profile of CGA compounds and metabolites in human plasma and urine after the acute consumption of a decaffeinated green coffee extract and to estimate the apparent bioavailability of CGA in this food matrix. Subjects and Methods Subjects. We recruited nonsmoking male (n ¼ 5) and female (n ¼ 5) participants (22–55 y old) by word of mouth among students and faculty at the Universidade Federal do Rio de Janeiro. They were healthy as judged by a medical questionnaire, with normal blood values for hemoglobin and hematocrit, and were not taking any medication or nutri- tional supplements. The study protocol was approved by the Ethical Committee of Clementino Fraga Filho University Hospital at Universidade 1Supported by Actifs Innovant Department, Naturex S. A. (France) and CNPq (Brazil).2Author disclosures: A. Farah, M. Monteiro, C. Donangelo, and S. Lafay, no conflicts of interest.5Abbreviations used: AUC, area under the curve; CFQA, caffeoylferuloylquinicacid; CGA, chlorogenic acid; Cmax, maximum plasma concentration; CQA,caffeylquinic acid; diCQA, dicaffeoylquinic acid; FQA, feruloylquinic acid;p-CoQA, p-coumaroylqunic acid; Tmax, time corresponding to maximum plasmaconcentration. American Society for Nutrition. Manuscript received 3 July 2008. Initial review completed 28 July 2008. Revision accepted 2 September 2008. 2309doi:10.3945/jn.108.095554.by guest on July 6, 2012 jn.nutrition.org Downloaded from Federal do Rio de Janeiro and fully explained to the subjects who gavetheir written informed consent prior to participation.Green coffee extract. A hydroalcoholic decaffeinated green coffee extract produced from C. canephora cv. Pierre beans was spray-dried and encapsulated (0.2 g in each capsule; SVETOL).Study design and sample collection. Subjectswere instructed to avoid consumption of phenolic-containing foods for the 48 h prior to the study. Theywere asked to eat only animal foods, refinedcereal foods, andartificial beverages. On the day of the study, after 10–12 h overnight fasting, an i.v.catheter was inserted into the antecubital vein and a baseline heparinized blood sample was obtained. Two capsules of green coffee extract were offered to each subject and sequential blood drawswere obtained 0.5, 1, 2,3, 4, 5, 6, 7, and 8 h after the capsule consumption. Blood samples were collected into heparin-containing tubes. Baseline blood aliquots were used to determine hematocrit and hemoglobin levels by standard methods.Plasma samples were obtained by centrifugation of the blood samples immediately after being drawn. Urine samples were also collected at baseline interval (minus 2–0 h) and at intervals of 0–2 h, 2–4 h, 4–6 h, and 6–8 h after coffee consumption into appropriate plastic containers. Total urine volume was measured for each collection period. Plasma and urine aliquots for determination ofCGAwere acidifiedwithHCl and kept frozen in liquid nitrogen until analyses. Urine aliquots for determination of creatininewere acidifiedwithHCl and kept at220[1]Cuntil analyses. Every hour, starting 1 h after green coffee extract consumption, subjects ate a CGA-free snack composed of white bread (25 g) with cream cheese (15 g) and 100 mL of a saline solution containing 0.21 g of NaCl, 2.28 g of glucose, 0.22 g of potassium citrate monohydrate, and 0.1 g of sodium citrate dihydrate, until the end of blood draws.Blood hemoglobin, hematocrit, and urinary creatinine. Hematocrit was determined by conventional capillary centrifugation. Blood hemo- globin was measured by the cyanomethemoglobin method, using a com-mercial kit (BioClin). Urinary creatinine was measured by the Jaffe reaction, as previously described (16).Chromatographic analyses. Analyses of CGA (including CGA lactones and caffeoyltryptophan) in the green coffee extract, plasma, and urine were performed by HPLC and LC-Diode Array Detector-MS gradient systems as described in detail by Farah et al. (14,17) andMonteiro et al. (15). The detection limit for 5-CQA (4-fold baseline noise) under the conditions used in this study was 0.01 mg/L. Urinary CGA and phenolic acids excretions were expressed relative to that of creatinine (15). Molar ratios of specific CGA compounds were calculated in green coffee extract as ratios of total amounts and, in plasma, as ratios of the corresponding area under the curve (AUC).Pharmacokinetics and apparent bioavailability calculations. For each subject, plasma concentrations of cinnamic acids and of individual and total CQA, diCQA, and CGA compounds were plotted over 8 after consumption of the green coffee extract. From this plotting, the following plasma pharmacokinetic parameters were calculated: AUC (mmol[1]h[1]L21) of total and individual components, using the trapezoidal approach (GraphPad Prism software, version 4.0); maximum plasma concentration (Cmax)(mmol/L) and time corresponding to Cmax (Tmax) (h) of total and individual components. Molar ratios between AUC of specific components were also calculated. For calculation of the apparent bioavailability of green coffee extract CGA based on plasma results, the body surface area was initially estimated as follows: SurfaceArea ¼ ðWeight 0:425 3Height 0:725 Þ371:84 10000; where the subject’s weight is given in kg and the height is given in cm. Surface area was used to obtain the corresponding red cell mass for the respective gender and to estimate the total blood volume as described by Frenkel et al. (18). Plasma volume was estimated as follows:PlasmaVolume ðmLÞ¼ BloodVolume ðmLÞ3 12Hematocrit 100: Finally, the plasma amount of CGA and cinnamic acids for the 8 h after green coffee consumption was calculated by multiplying the AUC of total CGA and cinnamic acids by the total plasma volume. The apparent bioavailability for total CGA was estimated as follows:Apparent Bioavailability%¼ PlasmaCGAðmmolÞ1Cinnamic acids ðmmolÞ CGAConsumed ðmmolÞ3100: Urinary recovery calculations were made considering the total number of equivalent moieties of cinnamic and quinic acids consumed in the green coffee extract and the total number of phenolic acid moieties recovered in urine, as a percentage.Statistical analyses. Results are means with corresponding SD. Asso-ciations between plasma AUC or Cmax and urinary excretion of specificcompounds were tested using Spearman correlations (GraphPad Prism).Differences were considered significant at P # 0.05. Results Green coffee extract. Nine major and 14 minor CGA compounds were identified in the decaffeinated green coffee extract offered to the subjects. The 2 capsules (0.4 g) consumed on the test day contained 170mg (451 mmol) of CGA (including lactones and cafeoyltryptophan) (Table 1). CQA represented 71.2%of CGA in the capsules, with 5-CQA, 4-CQA, and 3-CQA contributing to 26.6, 21.6, and 23% (wt:wt), respectively. DiCQA and FQA represented 9.6 and 13.2% of CGA compounds, respectively.Subject characterization. Subjects were healthy and had normal blood biochemistry and BMI (Table 2).Plasma samples. At baseline, 9 of 10 subjects had small amounts of 3-CQA, 4-CQA, 5-CQA, 3,4-diCQA, 3,5-diCQA, 4,5-diCQA,TABLE 1 Contents of the main CGA in 2 capsules of thedecaffeinated green coffee extract
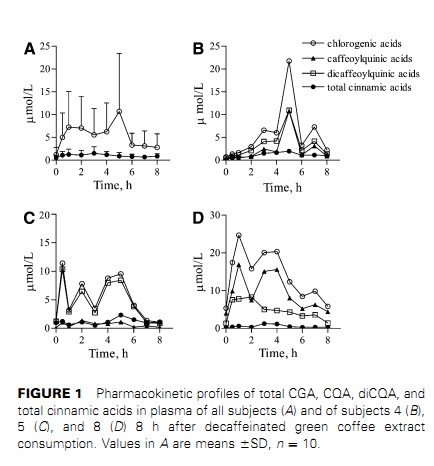
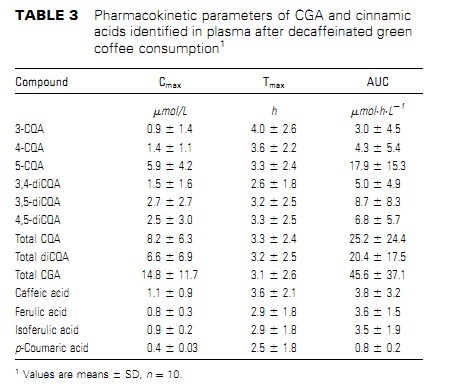
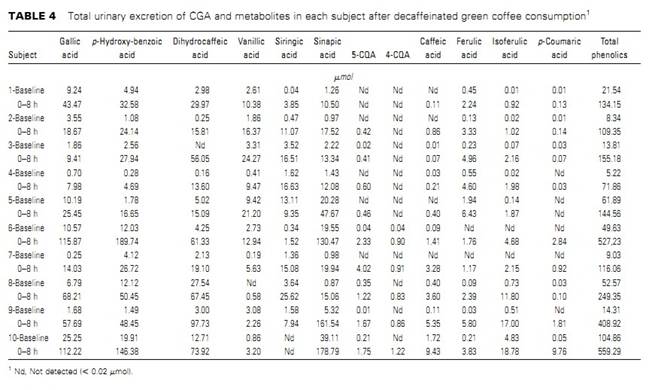
Caffeic acid derivatives computed as CGA.2310 Farah et al.by guest on July 6, 2012 jn.nutrition.org Downloaded from and caffeic acid in plasma. Ferulic acid was identified in 6 subjects, isoferulic acid in 5 subjects, and p-coumaricacidin3subjects.After green coffee extract consumption, 3-CQA, 4-CQA, 5-CQA,3,4-diCQA, 3,5-diCQA, and 4,5-diCQA were identified in the plasma of all subjects. Together, these compounds comprised;82% of the total CGA in the green coffee extract. No FQA was detected in plasma of any subjects before or after extract con-sumption. Caffeic, ferulic, isoferulic, and p-coumaric acids, which were not detected in the encapsulated extract, were present in the plasma of different subjects after green coffee extract consumption,contributing 6.6, 6.2, 6.1, and 1.4% of total phenolics in plasma,respectively. A large inter-individual variation was observed in the phar-macokinetic profile of all CGA compounds and cinnamic acids in plasma (Fig. 1). However, the plasma kinetic profiles of the individual isomers within the subclasses of CGA followed a similar pattern for most subjects. Therefore, for clarity, only the plasma pharmacokinetic profiles of total CQA, total diCQA,total CGA, and total cinnamic acids at baseline and for 8 h after coffee consumption are shown. CGA Cmax and Tmax varied substantially among the subjects.The Cmax of total CQAvaried from 0.6 to 16.9 mmol/L and that of total diCQA varied from 0.3 to 22.8 mmol/L, whereas the Cmax of total CGA varied from 1.2 to 39.7 mmol/L. Tmax for total CQA, total diCQA, and total CGA varied from 0.5 to 8 h (Table 3). The Tmax of the isomers did not differ within the CQA and diCQA subclasses.Of the individual compounds, 5-CQA was the major CGA identified in the plasma of all subjects at all time points after green coffee extract consumption, as indicated by both its Cmax and AUC. Based on AUC, 5-CQA, 4-CQA, and 3-CQA com-prised 31.3, 7.5, and 5.2% of the total phenolic compounds in plasma. Molar ratios among CGA compounds were calculated using their content in the green coffee extract and the AUC in plasma. For CQA, the ratio of 5-CQA:4-CQA:3-CQA in the green coffee extract was 1.2:1.0:1.1, whereas their corresponding ratio in plasma was 6.0:1.4:1.0. The molar ratio of 3,5-diCQA:4,5-diCQA:3,4-diCQA in the coffee extractwas 1.0:1.6:1.7,where as in plasma is was 1.7:1.4:1.0.Moreover, when the CGA classes were compared, the diCQA:CQA molar ratio in plasma was 6.2 times that in the green coffee extract (Tables 1 and 3).Urine samples.Most subjects had phenolic compounds in their urine at baseline. Trace amounts of 5-CQA were observed in 5 subjects. Sinapic, gallic, p-hydroxybenzoic, and dihydrocaffeic acids were the major phenolic compounds at baseline, representing ;82% of the total amount of the identified phenolic compounds. The urinary excretion of phenolic compounds increased in 9 of 10 subjects after extract consumption (Table 4).As with plasma, there was a large inter-individual variation in the urinary excretion of all compounds after green coffee extract consumption. The only intact CGA compounds identified in urine after the extract consumption were 5-CQA and 4-CQA. Not only at baseline but also after extract consumption, sinapic, gallic, p-hydroxybenzoic, and dihydrocaffeic acidswere themajor phenolic compounds, representing ;85% of the total amount of phenolic compounds identified in urine. Protocatechuic, dihydroferulic, benzoic, and hippuric acids, which have been previously identified in urine after CGA consumption, were not
Apparent bioavailability of CGA. As with plasma and urinary values, the apparent bioavailability of CGA varied considerably among subjects. The apparent bioavailability based on consumed cinnamic moieties ranged from 7.8 to 72.1% in plasma,with a mean of 33.1 6 23.1%. Urinary recovery of phenolic compounds based on consumed cinnamic and quinic acid moieties varied from 215.6 to 25.9% in urine when expressed as an increment from baseline values and from 7.5 to 58.4% when expressed as absolute values, with means of 5.5 6 10.6% and 25.8 6 19.1%, respectively. There was a negative correlation between apparent bioavailability and urinary recovery of CGA (r ¼ 20.76; P ¼ 0.01). Apparent bioavailability and urinary recovery were not correlated with gender, age, weight,height, or BMI. Discussion This is the first study, to our knowledge, evaluating the pharmacokinetics of CGA compounds after the consumption of a green coffee extract. Also, the apparent bioavailability of CGA compounds from a food matrix and the urinary recovery of their metabolites were estimated in humans. Although subjects consumed a low-phenolic diet for 2 d prior to the study and were fasting for 10–12 h when baseline samples were collected, all subjects had phenolic compounds in their baseline plasma and/or urine. This is in agreement with the fact that CGA and other phenolic compounds have been observed in saliva, gastrointestinal fluids, and urine of fasting subjects (19–21) and corroborates the hypothesis of storage and recycling of these compounds through excretion and reabsorption suggested by Baer-Dubowska and Szaefer (22) and Farah et al. (20).After green coffee extract consumption, 6 major CGA compounds were identified in the plasma of all subjects, as previously observed after roasted coffee consumption (15), accounting for almost 90% of phenolic compounds in plasma. Despite similar amounts of FQA and diCQA isomers in the extract, FQA was not detected in the plasma of any subject. This result is consistent with evidence of a poor absorption of FQA and/or of a rapid uptake by organs such as liver (23,24) and adipose tissue. In fact, a human hepatic cell study found that FQA uptake was favored compared with CQA and diCQA uptake (24).Another possible explanation would be demethylation of the ferulic acid moiety of the ester and conversion of FQA into CQA(Fig. 2). Additionally, small amounts of caffeic, ferulic, isoferulic, and p-coumaric acids were identified in the plasma of different subjects after the green coffee extract consumption. Considering that no unesterified cinnamic acids were present in the extract,that only 4.4% of 5-CQA were hydrolyzed into caffeic acid in the analytical recovery tests, and that isoferulic acid could not be formed from CGA hydrolysis, our results suggest that most cinnamic acids in plasma originated from metabolization of CGA from the coffee extract, possibly in the intestinal lumen/ mucosa and liver (28,33). Ferulic and isoferulic acids may be formed by methylation of caffeic acid (27). Conversely, caffeicacid may also derive from hydrolysis of FQA (34) (Fig. 2). As previously observed after roasted coffee consumption(15), there was a large inter-individual variation in the pharmacokinetic profile of all CGA compounds and phenolic acids in plasma and urine after green coffee extract consumption,although the kinetic profiles of the individual CGA compounds within the subclasses were similar for each subject. This variability may be attributed to inter-individual differences in TABLE 4 Total urinary excretion of CGA and metabolites in each subject after decaffeinated green coffee consumption1
Nd, Not detected (, 0.02 mmol).2312 Farah et al.by guest on July 6, 2012 jn.nutrition.org Downloaded from digestive transit time, preferential site of absorption, and metabolism of cinnamates as reported in the literature for other phenolic compounds (15,20,35). According to Manach et al.(35), important inter-individual differences in the capacity to metabolize phenolic compounds originate from differences in the activity of the cytochrome P450 and carrier systems that may be influenced by genetic polymorphisms. In contrast to the pharmacokinetic profile of flavonoids such as catechin (36) and isoflavones (37), various plasma concen- tration peaks were observed from 0.5 up to 8 h after the green coffee extract consumption (Fig. 1), suggesting a complex and dynamic process of absorption and metabolism. This irregular pharmacokinetic pattern makes the calculation of the CGA compounds half-life difficult. Longer duration studies would be necessary for this measurement. Considering that liquid food may take up to 1 h to reach the small intestine (38,39) and that preliminary tests showed that the extract capsules take ,15 min to solubilize in a simulated gastric condition (data not shown), the initial plasma concentration peaks confirm an early absorption of CGA in the stomach and jejunum followed by absorption along the small intestine (15,40–43). The later plasma peaks (.5 h) in some subjects may indicate absorption through the large intestine (44) and/or recycling through digestive fluids (20).In the present study, the higher diCQA:CQA molar ratio in plasma compared with the green coffee extract supports previous evidence of differential mechanisms of absorption and/or metabolism for CQA and diCQA (15), with favored tissue uptake of CQA compared with diCQA and/or favored diCQA absorption that could be related to the higher lipophilicity of diCQA compared with CQA, possibly favoring diCQA diffusion through the intestinal mucosa cells. Acylmigration from CQA forming diCQA during analysis is not possible, because adding green coffee extract to CGA-free plasma in a recovery test yielded similar CQA and diCQA isomer profiles in both the spiked plasma and the green coffee extract alone.The higher mean molar ratio of 5-CQA:4-CQA and 3-CQA in plasma than in green coffee extract supports preferential absorption of 5-CQA compared with 4-CQA and 3-CQA isomers or of rapid metabolization and/or uptake of 3-CQA and 4-CQA by organs such as liver (24,15) and adipose tissue. The highermolar ratios of 3,4-diCQA:4,5diCQA and 3,5diCQA in plasma than in the green coffee extract also suggests mechanisms favoring plasma levels of 3,5-diCQA as described for5-CQA. Although the amount of CGA in decaffeinated green coffee in the present study was ;13% of that in decaffeinated roasted coffee in our previous study (15), the plasma total CGA AUC in the present study at 4 h after treatment, the duration of our previous study, was 1.4 times the AUC in that study. Although the 2 studies cannot be compared directly due to various differences, including subjects, the influence of the matrix in which CGA is consumed deserves investigation. The 4 major urinary phenolic compounds excreted after green coffee consumption (sinapic, gallic, p-hydroxybenzoic, and dihydrocaffeic acids) are probably preferential metabolic products of the cinnamates identified in plasma. Vanillic and siringic acids are probably derived from a secondary metabolic pathway (Fig. 2). Protocatechuic, dihydroferulic, benzoic, and hippuric acids, which were identified in studies evaluating the urinary products of cinnamates from different food sources (25,27), were not identified in our study although they are consistent with the metabolic pathway presented (Fig. 2). This is the first study to our knowledge investigating the bioavailability of CGA as a family of compounds and also the FIGURE 2 Proposed simplified scheme of 5- CQA metabolism, as representative of CQA and diCQA classes, based on results from the present study. Support references for proposed metabolic routes are shown. The authors adopted the IUPAC numbering system (32) for CGA.Chlorogenic acids bioavailability in humans 2313 by guest on July 6, 2012 jn.nutrition.org Downloaded from first evaluating the bioavailability of CGA using plasma AUC values. The apparent bioavailability of CGA from the green coffee extract varied from 7.8 to 72.1% among the subjects,with a mean of 33 6 23%. Possibly, a higher bioavailability would have been obtained in a longer study, because in 3 of the subjects, plasma CGA concentrations were still high 8 h after green coffee extract consumption. The mean apparent bioavail-ability of 5-CQA, the only CGA evaluated in previous bioavail-ability studies, was 33 6 27%. This result is in contrast with the very low absorption of 5-CQA estimated in rat and human studies (as low as 0.1%) (42,45,46). On the other hand, our results are in agreement with the 33 6 17% absorption estimated by Olthof et al. (47), who analyzed ileostomy fluids from colostomized subjects for 24 h after the consumption of 2.8 mmol of 5-CQA. The results by Olthof et al. were considered an overestimation (48), because the amount of 5-CQA possibly lost during digestion was not taken into account and because previous studies (21,48,49) had been unable to identify intact CGA in plasma. However, the results obtained by Olthof et al.(47) are actually very close to those in the present study and their estimate was probably correct, because only a small portion of 5-CQA seems to be hydrolyzed in the digestive tract and only a minor fraction is absorbed in the formof caffeic acid (15, presentstudy). The low recovery of total phenolic compounds in urine when values were expressed as increments from baseline was down-graded by the high amount of phenolic compounds in the baseline urine period of 1 subject (subject 8; Table 4), producing a negative recovery of 215.6%. Excluding this subject would bring the recovery, corrected to baseline, to 7.8 6 8.1%. This case, along with the irregular pharmacokinetic patterns observed in plasma, indicates that increments over baseline values may not be the most appropriate way to evaluate total urinary excretion of CGA andmetabolites in response to acute ingestion. On the other hand, the use of absolute urinary values (not corrected by baseline) may overestimate excretion. Low recoveries in urine were also found in previous human studies evaluating the metabolism of phenolic compounds, supporting evidence that urine may not be the preferential excretion route for intact CGA and metabolites (19,20,26,50). On the other hand, it should be noted that many urinary metabolites derived from CGA were probably not identified in this study, because the 12 urinary compounds identified represented ;50% of the total chromatogram peak area.Olthof et al. (25) identified 23 phenolic acids by GC in human urine after consumption of 5-CQA. The negative correlation between apparent bioavailability of CGA and corrected urinary recovery (r ¼ 20.76; P ¼ 0.01) may be explained by inter-individual differences in metabolic rates and/or preferential excretion routes. In conclusion, the present study confirms that CQA and diCQA, which are major CGA compounds in coffee, are absorbed in the human body, being differentially absorbed and/or metabolized throughout the whole gastrointestinal tract. This study also supports evidence that urine is not a major excretion pathway of intact CGA compounds and their metabolites and identifies sinapic, gallic, p-hydroxybenzoic, and dihydrocaffeic acids as major urinary metabolites of CGA in humans. In addition, this study shows that the main CGA compounds present in the green coffee matrix are highly bioavailable in humans. A large inter-individual variation clearly exists in CGA absorption, metabolism, and kinetics in humans and requires further investigation into differences in genetic polymorphisms. Literature Cited 1. Ranheim T, Halvorsen B. Coffee consumption and human health: beneficial or detrimental? Mechanisms for effects of coffee consumption on different risk factors for cardiovascular disease and type 2 diabetes mellitus. Mol Nutr Food Res. 2005;49:274–84. 2. Salazar-Martinez E, Willett WC, Ascherio A, Manson JE, Leitzmann MF, Stampfer MJ, Hu FB. Coffee consumption and risk for type 2 diabetes mellitus. Ann Intern Med. 2004;140:1–8. 3. Lindsay J,LaurinD,VerreaultR,HebertR,HelliwellB,HillGB,McDowell I. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study ofHealth and Aging. AmJ Epidemiol. 2002;156:445–53. 4. Almeida AA, Farah A, Silva DAM, Nunam EA, Glo ´ ria MBA. Antibac- terial activity of coffee extracts and selected coffee chemical compounds against enterobacteria. J Agric Food Chem. 2006;54:8738–43. 5. Santos MD, Almeida MC, Lopes NP, Souza GEP. Evaluation of the antiinflamatory, analgesic and antypiretic activity of the natural poly- phenol chlorogenic acid. Biol Pharm Bull. 2006;29:2236–40. 6. Shearer J, Farah A, de Paulis T, Bracy DP, Pencek RR, Graham TE, Wasserman DH. Quinides of roasted coffee enhance insulin action in conscious rats. J Nutr. 2003;133:3529–32. 7. Farah A, Donangelo CM. Phenolic compounds in coffee. Braz J Plant Physiol. 2006;18:23–36. 8. Suzuki A, Kagawa D, Ochiai R, Tokimitsu I, Saito I. Green coffee bean extract and its metabolites have a hypotensive effect in spontaneously hypertensive rats. Hypertens Res. 2002;25:99–107. 9. Kozuma K, Tsuchiya S, Kohori J, Hase T, Tokimitsu I. Antihypertensive effect of green coffee bean extract on mildly hypertensive subjects. Hypertens Res. 2005;28:711–8. 10. Ochiai R, Jokura H, Suzuki A, Tokimitsu I, OhishiM, Komai N, Rakugi H, Ogihara T. Green coffee bean extract improves human vasoreactivity. Hypertens Res. 2004;27:731–7. 11. Shimoda H, Seki E, Aitani M. Inhibitory effect of green coffee bean extract on fat accumulation and body weight gain in mice. BMC Com- plement Altern Med. 2006;6:1–9. 12. Dellalibera O, Lemaire B, Lafay S. Le Svetol, un extrait de cafe ´ vert de ´cafe ´ ine ´ , induit une perte de poids et augument le ratio masse maigre sur masse grasse chez des volontaires en surcharge ponde ´rale. Phytother- apie. 2006;4:1–4. 13. Blum J, Lemaire B, Lafay S. Effect of a green decaffeinated coffee extract on glycaemia. NutraFoods Res. 2007;6:13–7. 14. Farah A, De Paulis T, Trugo LC, Martin PR. Effect of roasting on the formation of chlorogenic acid lactones in coffee. J Agric Food Chem. 2005;53:1505–13. 15. MonteiroM, Farah A, Perrone D, Trugo LC, Donangelo C. Chlorogenic acid compounds from coffee are differentially absorbed and metabo- lized in humans. J Nutr. 2007;137:2196–201. 16. Husdan H, Rapoport A. Estimation of creatinine by the Jaffee reaction. A comparison of three methods. Clin Chem. 1968;14:222–38. 17. Farah A, De Paulis T, Moreira DP, Trugo LC, Martin PR. Chlorogenic acids and lactones in regular and water-decaffeinated arabica coffees. J Agric Food Chem. 2006;54:374–81. 18. Frenkel EP, McCall MS, Reisch JS, Minton PD. An analysis of methods for the prediction of normal erythrocyte mass. Am J Clin Pathol. 1972; 58:260–71. 19. Farah A. Distribuic xa ˜o nos gra ˜os, importa ˆncia na qualidade da bebida e biodisponibilidade dos a ´cidos cloroge ˆncios do cafe ´ [PhD thesis]. Rio de Janeiro: Universidade Federal do Rio de Janeiro; 2004. 20. Farah A,MonteiroMC, Guigon F,Moreira DP, Donangelo CM, Trugo LC. Chlorogenic acids from coffee are absorbed and excreted in human digestive fluids. Proceedings of the 21st International Conference on Coffee Science. 2006:58–64. 21. Cremin P, Kasim-Karakas S, Waterhouse AL. LC/ES-MS detection of hydroxycinnamates in human plasma and urine. J Agric Food Chem. 2001;49:1747–50. 22. Baer-Dubowska W, Szaefer H. Inhibition of murine hepatic cytochrome p-450 acitivies by natural and synthetic compounds. Xenobiotica. 1998; 28:735–43. 23. Booth AN, Emerson OH, Jones FT, Deeds F. Urinary metabolites of caffeic and chlorogenic acids. J Biol Chem. 1957;229:51–9. 24. Farah A, Trugo LC. Chlorogenic acid isomers from coffee are differ- entially uptaken by HEPG2 human hepatocite cell line. Proceedings of the 21st International Conference on Coffee Science. 2006:101–4. 2314 Farah et al. by guest on July 6, 2012 jn.nutrition.org Downloaded from 25. Olthof MR, Hollman PCH, Buijsman MNCP, Van Amelsvoort JMM, Katan MB. Chlorogenic acid, quercetin-3-rutinoside and black tea phenol are extensively metabolized in humans. J Nutr. 2003;133: 1806–14. 26. Choudhury R, Srai SK, Debnam E, Rice-Evans C. Urinary excretion of hydroxycinnamates and flavonoids after oral and intravenous admin- istration. Free Radic Biol Med. 1999;27:278–86. 27. Rechner AR, Spencer JPE, Kuhnle G, Harn U, Rice-Evans CA. Novel biomarkers of the metabolism of caffeic acid derivatives in vivo. Free Radic Biol Med. 2001;30:1213–22. 28. Kern SM, Bennett RN, Needs PW, Mellon FA, Kroon PA, Garcia- Conesa MT. Characterization of metabolites of hydroxycinnamates in the in vitro model of human small intestinal epithelium Caco-2 cells. J Agric Food Chem. 2003;51:7884–91. 29. Booth AN, Masri MS, Robbins DJ, Emerson OH, Jones FT, DeEds F. The metabolic fate of gallic acid and related compounds. J Biol Chem. 1959;234:3014–6. 30. Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–56. 31. Dec J, Haider K, Bollag JM. Release of substituents from phenolic compounds during oxidative coupling reactions. Chemosphere. 2003; 52:549–56. 32. IUPAC. Nomenclature of cyclitols. Biochem J. 1976;153:23–31. 33. Farah A, Guigon F, Trugo LC. The effect of human digestive fluids on chlorogenic acid isomers in coffee. Proceedings of the 21st International Conference on Coffee Science. 2006:93–6. 34. Bourne LC, Rice-EvansC. Bioavailability of ferulic acid. BiochemBiophys Res Commun. 1998;253:222–7. 35. Manach C, Scalbert S, Morand C, Re ´me ´sy C, Jime ´nez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47. 36. Masukawa Y,Matsui Y, Shimizu N, Kondou N, Endou N, KuzukawaM, Hase T. Determination of green tea catechins in human plasma using liquid chromatography–electrospray ionization mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;834:26–34. 37. Pascual-Teresa ST, Hallund J, Talbot D, Schroot J,Williams CM, Bugel S, Cassidy A. Absorption of isoflavones in humans: effects of food matrix and processing. J Nutr Bioch. 2006;17:257–64. 38. Minami H, McCallum RW. The physiology and pathophysiology of gastric emptying in humans. Gastroenterology. 1984;86:1592–610. 39. Moore JG, Christian PE, Coleman RE. Gastric emptying of varying meal weight and composition in man. Evaluation by dual liquid and solid-phase isotopic method. Dig Dis Sci. 1981;26:16–22. 40. Lafay S, Gil-Izquierdo A, Manach C, Morand C, Besson C, Scalbert A. Chlorogenic acid is absorbed in its intact form in the stomach of rats. J Nutr. 2006;136:1192–7. 41. Konishi Y, Zhao Z, ShimizuM. Phenolic acids are absorbed from the rat stomach with different absorption rates. J Agric Food Chem. 2006; 54:7539–43. 42. Spencer JP, Chowrimootoo G, Choudhury R, Debnam ES, Srai SK, Rice-Evans C. The small intestine can both absorb and glucuronidate luminal flavonoids. FEBS Lett. 1999;458:224–30. 43. Lafay S, Morand C, Manach C, Besson C, Scalbert A. Absorption and metabolism of caffeic acid and chlorogenic acid in the small intestine of rats. Br J Nutr. 2006;96:39–46. 44. Dupont MS, Bennett RN, Mellon FA, Williamson G. Polyphenols from alcoholic apple cider are absorbed, metabolized and excreted by humans. J Nutr. 2002;132:172–5. 45. Dupas C, Baglieri AM, Ordonaud C, Tom D, Maillard M. Chlorogenic acid is poorly absorbed, independently of the food matrix: a Caco-2 cells and rat chronic absorption study. Mol Nutr Food Res. 2006; 50:1053–60. 46. Bourne LC, Rice-Evans CA. Urinary detection of hydroxycinnamates and flavonoids in humans after high dietary intake of fruit. Free Radic Res. 1998;28:429–38. 47. Olthof MR, Hollman PCH, Katan MB. Chlorogenic acid and caffeic acid are absorbed in humans. J Nutr. 2001;131:66–71. 48. Nardini M, Cirillo E, Natella F, Scaccini C. Absorption of phenolic acids in humans after coffee consumption. J Agric Food Chem. 2002; 50:5735–41. 49. Azuma K, Ippoushi K, Nakayama M, Ito H, Higashio H, Terao J. Absorption of chlorogenic acid and caffeic acid in rats after oral ad- ministration. J Agric Food Chem. 2000;48:5496–500. 50. Gugler R, Leschik M, Dengler HJ. Disposition of quercetin in man after single oral and intravenous doses. Eur J Clin Pharmacol. 1975; 9:229–34. Chlorogenic acids bioavailability in humans 2315 by guest on July 6, 2012 jn.nutrition.org Downloaded from |







 Chlorogenic Acids from Green Coffee Extract are Highly Bioavailable in Humans1,2
Chlorogenic Acids from Green Coffee Extract are Highly Bioavailable in Humans1,2