| Chlorogenic acid from the Japanese herbal medicine |
| 发布时间:2012-07-18 信息来源:admin 发布人:admin 点击次数:9392 |
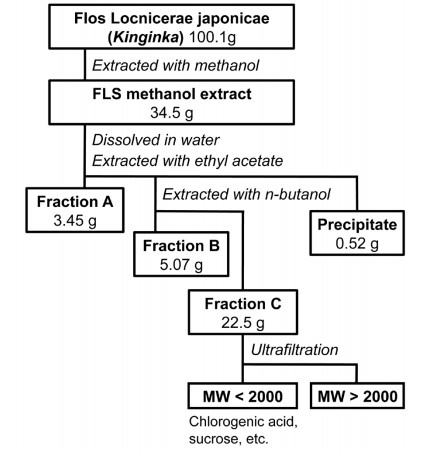
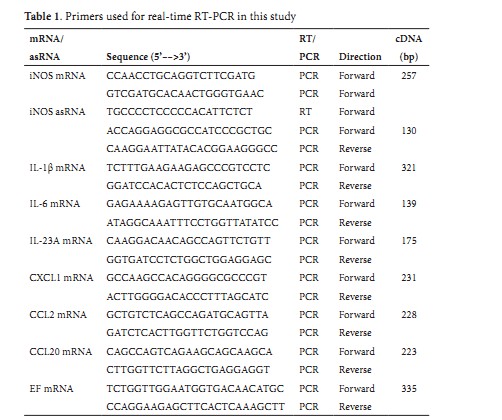
Chlorogenic acid from the Japanese herbal medicine Kinginka (Flos Lonicerae japonicae) suppresses the expression of inducible nitric oxide synthase in rat hepatocytes Abstract Background: Flos Lonicerae japonicae (FLJ; Kinginka) is the dried flowers and buds of the Japanese honeysuckle Lonicera japonica Thunberg. FLJ has been used as a Japanese Kampo medicine to treat infectious and inflammatory diseases. However, it is not clear which constituent of FLJ is responsible for its pharmacological effects. Methods: FLJ was extracted with methanol and fractionated by hydrophobicity. We measured the effects of each fraction on the induction of the inflammatory mediator nitric oxide (NO), which was induced by interleukin 1β in primary cultured rat hepatocytes. To estimate cytotoxicity, the activity of lactate dehydrogenase released from the hepatocytes was measured. The expression of inducible nitric oxide synthase (iNOS) was analyzed by Western blot analysis and reverse transcription-polymerase chain reaction. Results: The methanol extract was fractionated into hydrophobic (11.1%), butanol-soluble (16.4%), and water-soluble fractions (72.5%). These three fractions dose-dependently suppressed the induction of NO and reduced the level of iNOS protein in interleukin 1β-stimulated hepatocytes. Chlorogenic acid, a major constituent of the water-soluble fraction, significantly reduced the levels of NO production, iNOS protein, and iNOS mRNA. Chlorogenic acid also decreased the levels of mRNAs encoding cytokines and chemokines that are involved in inflammatory disease. Caffeic acid, which is formed by the hydrolysis of chlorogenic acid, markedly reduced the induction of NO, although it did not exist at a detectable level in the water-soluble fraction. In contrast, other constituents of the water-soluble fraction, such as inositol fructose, glucose, and sucrose, did not affect the induction of NO. Conclusions: The anti-inflammatory effects of the FLJ extract and its constituents were analyzed by measuring the induction of NO and iNOS in hepatocytes. We demonstrated that chlorogenic acid, one of the main constituents of FLJ, is involved in the anti-inflammatory effect of the FLJ extract, suggesting its therapeutic potential. © 2012 Nishizawa et al; licensee Herbert Publications Ltd. This is an open access article distributed under the terms of Creative Commons Attribution License,This permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly citedCorrespondence: Department of Biomedical Sciences, College of Life Sciences, Ritsumeikan University, 1-1-1 Nojihigashi, Kusatsu, Shiga 525-8577, Japan. Full list of Author’s information is available at the end of the article Background The Japanese honeysuckle Lonicera japonica Thunberg is a vine shrub that commonly grows in Japan, Korea, and China, and flowers of Lonicera japonica are sweetly scented and produce nectar [1]. The flowers are white at first and then become yellow. Flos Lonicerae japonicae (FLJ; Kinginka in Japanese), the dried flowers and buds of Lonicera japonica, is included in various prescriptions of Japanese Kampo medicine to treat infectious and inflammatory diseases due to its antiviral, antibacterial, anti-inflammatory, and antipyretic effects [2]. FLJ is popularly used also in China as a traditional medicine, such as Yin Hua tea and Jin Yin wine [2]. However, a mechanism of the pharmacological effects of FLJ is not well understood. More than 100 constituents have been isolated from the flowers, leaves, and stems of Lonicera japonica [2]. Some of the most prominent constituents of Lonicera japonica are chlorogenic acids, which is a family of esters formed between trans-cinnamic acids and quinic acid that is also abundant in coffee beans [2,3]. The most common chlorogenic acid is an ester of caffeic acid and quinic acid, also known as 5-O-caffeoylquinic acid. The contents of chlorogenic acid in the FLJ samples collected in different locations are determined to be 19.8–29.5 mg/g 50% methanol extract by high-performance liquid chromatography (HPLC) coupled with diode-array and evaporative light scattering detectors [4]. A few constituents involved in the pharmacological activity of Lonicera japonica have been reported. Luteolin, a flavonoid isolated from the flowers of Lonicera japonica, suppresses the expression of tumor necrosis factor α (TNF-α) and cyclo-oxygenase 2 (COX-2) in human mast cells [5]. In addition, the purified extract from the stems of Lonicera japonica, which are rich in loganin and sweroside, has anti-inflammatory and analgesic activity in mice [5]. However, luteolin, loganin, and sweroside are not major constituents of the Lonicera japonica extract [4]. It remains unclear which constituent in FLJ is primarily responsible for its anti-inflammatory activity and how the constituent suppresses inflammation. Not only TNF-α and COX-2 but also other molecules that are involved in inflammation remain to be investigated to understand the anti-inflammatory activity of FLJ.Herbal medicines generally consist of many compounds, and it is difficult to isolate and identify their pharmacologically active constituents. According to our data to purify more than 20 herbal medicines, cytotoxic compounds were often constituents of the total extract of herbal medicine, for example, the rhubarb Rhei Rhizoma [7]. Such cytotoxic compounds, including saponins and tannins, may mask the pharmacological activities of an herbal extract in Nishizawa et al. HOAJ Biology 2012, in vitro assays. Therefore, we established a standardized protocol to fractionate the herbal extract of FLJ by hydrophobicity and estimate the pharmacological activity of each fraction. We analyzed the anti-inflammatory effect of the FLJ extract on the induction of the inflammatory mediator nitric oxide (NO), which was induced by the pro-inflammatory cytokine interleukin 1β (IL-1β) in rat hepatocytes. The induction of NO and inducible nitric oxide synthase (iNOS) in the IL-1β-stimulated hepatocytes mimics liver injury [8,9]. We simultaneously estimated the cytotoxicity of the FLJ fractions by measuring the activity of lactate dehydrogenase (LDH) released from the injured hepatocytes. Furthermore, the FLJ extract may affect on expression of the cytokines and chemokines that are induced by IL-1β. In this study, we indicated that chlorogenic acid, the main constituent of the FLJ extract, suppresses induction of NO production and expression of the cytokines and chemokines that are involved in inflammation. We demonstrated that chlorogenic acid is the main constituent responsible for the anti-inflammatory activity of FLJ in hepatocytes and discuss the structure-activity relationship of chlorogenic acid and its metabolites with respect to their therapeutic potential. Materials & Methods Plant material, extraction, and fractionation (ABC fractionation): The flowers and buds of Lonicera japonica Thunberg, which were collected in Henan Province, China, and identified and authenticated by Dr. Yutaka Yamamoto (Tochimoto Tenkaido Co. Ltd., Osaka, Japan) and Professor Yukinobu Ikeya, a co-author of this paper, were purchased from Tochimoto Tenkaido Co. Ltd. The voucher specimen was deposited in the Ritsumeikan Herbarium of Pharmacognosy, Ritsumeikan University (Kusatsu, Shiga, Japan) under code number RIN-LJ-010. The dried flowers and buds (length, 3.0–11.0 mm; diameter, 0.9–1.4 mm) of Lonicera japonica (100.1 g) were extracted twice with absolute methanol under reflux for 1 h. The solvent was evaporated under reduced pressure, yielding the methanol extract. The resultant methanol extract was resuspended in water. After filtration of the suspension, it was successively partitioned with ethyl acetate and n-butanol. These layers were concentrated to give an ethyl acetate-soluble fraction (fraction A; hydrophobic), an n-butanol-soluble fraction (fraction B), and a water-soluble fraction (fraction C; hydrophilic) (Figure 1). Fraction C was further fractionated by ultrafiltration with a U-Tube Concentrator 2H-2 (Novagen, Madison, WI, USA) at a molecular weight (MW) cutoff of 2,000 Da, and the filtrate was collected by centrifugation at 1,600 × g for 9 h. The analysis of chlorogenic acid by HPLC: Quantitative analyses of chlorogenic acid in fraction C were performed using a Shimadzu LC-20A series HPLC instrument equipped with an SPD-20S detector at 254 nm (Shimadzu Corporation, Kyoto, Japan). Samples were separated by a Cosmosil Cholester column (4.6 mm internal diameter × 150 mm; Nacalai Tesque Inc., Kyoto, Japan) at 0.8 mL/min with a mobile phase of absolute acetonitrile:5% (v/v) acetic acid (10:90 to 50:50 over 35 min). Chlorogenic acid (Wako Pure Chemical Industries, Ltd., Osaka, Japan) was used as an analytical standard. The analysis of sugar composition by HPLC: The fraction C sample was dissolved in water and injected into a Cosmosil Sugar D column (4.6 mm internal diameter × 250 mm; Nacalai Tesque Inc.) equilibrated with 80% (v/v) acetonitrile at 1 mL/min delivered by a Shimadzu LC-20 AT (Shimadzu Corporation). Elution was monitored byrefractive index using a Shimadzu RID-10A. Data were collected and processed by a Shimadzu CR-7A. As analytical standards, fructose, glucose, sucrose, and myo-inositol (Wako Pure Chemical Industries, Ltd.) were used. Preparation of primary cultured rat hepatocytes: Hepatocytes were isolated from the livers of male Wistar rats (Charles River Laboratories Japan Inc., Yokohama, Japan) by collagenase perfusion as previously described [10]. Briefly, dispersed cells were centrifuged at 50 × g for 70 sec and further purified by centrifugation three times to remove non-parenchymal cells. The pellet was resuspended in Williams’ E medium (Sigma-Aldrich Corp., St. Louis, MO), seeded at 1.2 × 106cells/dish, incubated at 37°C for 2 h, and themedium was replaced twice with fresh medium containing 10% newborn bovine serum (SAFC Biosciences, Inc., Lenexa, KS, USA). The purity of the resultant hepatocytes was greater than 99% by microscopic observation (data not shown). The hepatocytes were incubated at 37°C overnight and treated with 1 nM rat IL-1β (PeproTech, Rocky Hill, NJ, USA) and a FLJ fraction or a compound. The animal experiments were approved by the Animal Care Committee of Ritsumeikan University, Biwako-Kusatsu Campus. Figure 1. A flowchart of the procedures to fractionate the FLJ extract.The fractionation procedures are schematically shown. The weights of the fractions and the constituents included in each fraction are indicated. The dried flowers and buds of Lonicera japonica (FLJ) were extracted with methanol and dried. This FLJ extract was sequentially fractionated by ethyl acetate (fraction A), n-butanol (fraction B), and water (fraction C). See also Table 2.Nishizawa et al. HOAJ Biology 2012 Determination of NO production and LDH activity: To indirectly measure the production of NO, triplicate measurements of nitrite (a stable metabolite of NO) by the Griess method [11] in the culture medium were performed, and the half-maximal inhibitory concentration (IC50) was determined in triplicate (n = 3 dishes per point) with at least three different concentrations. When a fraction or compound are not cytotoxic to hepatocytes, the NO levels at the concentrations are inversely proportional to log10[concentration] (i.e.,dose-dependent) and thus used to determine the IC50 values. As an indicator of cell viability and cytotoxicity, the LDH activity in the culture medium was measured, in triplicate (3 dishes per point), using LDH Cytotoxicity Detection Kits (Takara Bio Inc., Otsu, Japan). Caffeic, ferulic, isoferulic, and p-coumaric acids were purchased from Wako Pure Chemical Industries, Ltd. or Nacalai Tesque Inc. Western blot analysis: Total cell lysates were prepared essentially as described previously [12]. Briefly, cells (1 × 106 cells/35-mm dish) were lysed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (final concentrations of 125 mM Tris-HCl, pH 6.8, 5% glycerol, 2% SDS, and 2% 2-mercaptoethanol), subjected to SDS-PAGE and electroblotted onto a Sequi-Blot membrane (Bio-Rad, Hercules, CA, USA). Immunostaining was performed using primary antibodies against rat iNOS (Thermo Fisher Scientific, Waltham, MA, USA) and rat β-tubulin (internal control; Cell Signaling Technology, Inc., Danvers, MA, USA), followed by visualization with an Enhanced Chemiluminescence Blotting Detection Reagent (GE Healthcare Biosciences Corp., Piscataway, NJ, USA). Reverse transcription-polymerase chain reaction (RT-PCR): Total RNA was prepared from hepatocytes (2 dishes/point) using Sepasol I Super (Nacalai Tesque Inc.) and TURBO DNA-free kits (Applied Biosystems, Austin, TX, USA). The cDNA was reverse-transcribed in a strand-specific manner with an oligo(dT) primer and a sense (forward) primer for mRNA and as RNA, respectively [13]. Step-down PCR [14,15] was performed with paired primers (Table 1), and mRNA levels were estimated in triplicate by real-time PCR with SYBR Green I and the Thermal Cycler Dice Real Time System (Takara Bio Inc.), as described previously [13]. The values obtained were normalized to elongation factor 1α (EF) mRNA. Statistical analysis: The results in the figures and Table 2 are representative of at least three independent experiments yielding similar findings. Values are represented as the mean ± standard deviations (SD). Differences were analyzed using the Student’s t-test. Statistical significance was set at P < 0.05.
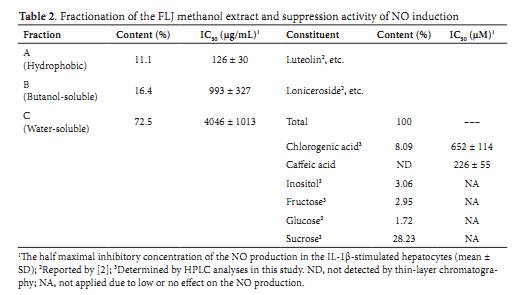
Results Extraction and fractionation of the FLJ extract: We extracted the flowers and buds of Lonicera japonica (FLJ) with methanol and fractionated this extract into three fractions based on hydrophobicity using ethyl acetate (fraction A), n-butanol (fraction B), and water (fraction C) sequentially (Figure 1). Generally, fraction A contained hydrophobic phenolic compounds, such as terpenoids, steroids, lignans, and flavonoids; fraction B contained tannins, saponins, and glycosides of the compounds in fraction A; and fraction C contained hydrophilic compounds, such as sugars, amino acids, and peptides. The proportions of the fractions by total solid weight were 11.1% hydrophobic fraction A, 16.4% butanol-soluble fraction B, and 72.5% water-soluble fraction C. Effects of the FLJ fractions on the induction of NO production: IL-1β induces iNOS expression, which is followed by the production of NO in primary cultured rat hepatocytes [8,9]. Thus, we used this system to monitor the anti-inflammatory activity of the FLJ extract. Fractions A, B, and C dose-dependently suppressed the induction of NO in IL-1β-stimulated hepatocytes (Figure 2A). With regard to the inhibition of NO production (Table 2), the activity of fraction A was the highest, with an IC50 value of 126 μg/mL. We next evaluated the release of LDH into the culture medium. All FLJ fractions showed no cellular cytotoxicity at the indicated concentrations (Figure 2B).Luteolin (3 ,4 ,5,7-tetrahydroxylflavone) is contained in Lonicera japonica flowers [5] and suppresses NO production in bacterial lipopolysaccharide (LPS)-stimulated macrophages [16]. Therefore, the NO suppression activity of fraction A might be at least partly contributed by luteolin. Given that fraction C was the majority of the FLJ extract, we examined the NO suppression activity of fraction C. For this purpose, fraction C was further purified by ultrafiltration at a MW cutoff of 2,000 Da. The NO suppression activity was present only in the filtrate (data not shown), suggesting that low-MW (< 2,000) constituents in fraction C may be responsible for the inhibition of NO induction in the IL-1β-stimulated hepatocytes. Chlorogenic acid suppresses the induction of NO and iNOS: Chlorogenic acid has a MW of 354.31 and is one of the main constituents of the Lonicera japonica plant [2]. Therefore, we investigated whether chlorogenic acid was included in fraction C. As shown in Figure 3A, pure chlorogenic acid (5-O-caffeoylquinic acid; showed a single peak at a retention time of 9.4 min on an HPLC chromatogram. When a sample of fraction C of the FLJ extract was loaded, a main peak at the same retention time was detected (Figure 3B). Using pure chlorogenic acid as a standard in HPLC analysis, we estimated that the content of chlorogenic acid in fraction C was 8.09% of the solid weight, i.e., 80.9 mg/g fraction C (Table 2). Thin-layer chromatography (TLC) of the fraction C sample also indicated the presence of chlorogenic acid in this fraction (data not shown). These data suggest that chlorogenic acid is also a main constituent of the flowers and buds of Lonicera japonica.Next, we investigated whether chlorogenic acid suppressed the induction of NO. Chlorogenic acid dose-dependently decreased the level of NO production, showing an IC50of 652 ± 114 μM (n = 4; 231 μg/mL). When comparing with the IC50 of fraction C (4046 μg/mL), chlorogenic acid is 17.5-fold more efficient than fraction C in suppressing NO induction. Chlorogenic acid was not cytotoxic to the hepatocytes within the indicated concentrations up to 3 mM, as evaluated by the release of LDH into the culture medium (data not shown). Western blot analysis revealed that chlorogenic acid dose-dependently decreased iNOS protein expression (Figure 4A). Real-time RT-PCR analyses revealed that chlorogenic acid reduced the expression of iNOS mRNA (Figure 4B). It has been previously shown that a natural antisense transcript (asRNA) is transcribed from the iNOS gene and stabilizes iNOS mRNA [13,17]. Chlorogenic acid also decreased the expression of iNOS asRNA (Figure 4B), implying that chlorogenic acid may reduce the stability of iNOS mRNA. Together, these data suggest that chlorogenic acid inhibits the induction of NO production and iNOS expression at the transcriptional and post-transcriptional steps.
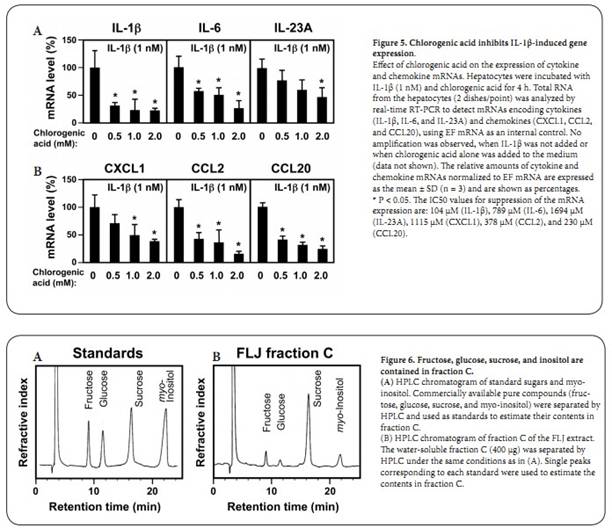
Chlorogenic acid inhibits the induction of cytokine and chemokine mRNAs: IL-1β, IL-6, and IL-23 p19 (IL-23A) are cytokines that play key roles in inflammatory and autoimmune disorders [18,19]. The chemokines CXCL1, CCL2, and CCL20 are also involved in inflammation [20,21]. To examine the effects of chlorogenic acid on the expression of these cytokines and chemokines, we performed real-time RT-PCR. As shown in Figure 5, the levels of the cytokine and chemokine mRNAs were increased by IL-1β stimulation. When chlorogenic acid was added, all of these mRNA decreased, suggesting that induction of the cytokine and chemokine mRNAs is suppressed by chlorogenic acid. Similarly to the inhibition of NO production (Table 2), we calculated the IC50 values for suppression of the mRNA induction. The IC50 values by chlorogenic acid vary from 104 μM (IL-1β) to 1694 μM (IL-23A), when comparing to that for the inhibition of NO production (652 μM). These results indicate that chlorogenic acid suppresses not only NO but also the cytokine/chemokine mRNA in a dose-dependent manner, although responsiveness to chlorogenic acid are different. Other low-MW constituents of the water-soluble fraction C: Because flowers of Lonicera japonica produce edible nectar [1], we speculated that they contain sugars and therefore estimated the content of sugars and inositol in fraction C shown in Figure 6. Our analysis indicated that fructose, glucose, and sucrose, as well as inositol, were included in fraction C. Among all the constituents examined in this study, the content of sucrose was the highest, i.e., 28.23% (w/w) in fraction C (Table 2). Next, we investigated the effects of these constituents on theinduction of NO production and iNOS expression by IL-1β. In contrast to chlorogenic acid, fructose, glucose, sucrose, or inositol did not affect the induction of NO production (Table 2) or iNOS expression (data not shown). Caffeic acid (3,4-dihydroxycinnamic acid) is a constituent of FLJ [2] and is formed by the hydrolysis of chlorogenic acid in the liver [22]. When fraction C was analyzed by TLC, caffeic acid was not detected (data not shown), suggesting that the content of caffeic acid was very low, or at least below the detection level of TLC. This result coincides with a report that traces or small amounts of caffeic acid are detected in the Lonicera japonica extract [4]. Unexpectedly, when pure caffeic acid was added to the medium, the NO induction was markedly suppressed in the hepatocytes (Table 2). The IC50value of caffeic acid (226 μM) was lower than that of chlorogenic acid (652 μM), indicating that the metabolite caffeic acid is 2.9-fold more efficient than chlorogenic acid in suppressing NO induction. Metabolites of chlorogenic acid suppress the induction of NO production: In the human liver and intestine, caffeic acid is methylated to yield ferulic and isoferulic acids or is converted to p-coumaric acid [22], as shown in Figure 7. Because the structures of these metabolites are similar to chlorogenic and caffeic acid, we analyzed the effects of these cinnamic acid derivatives on the suppression of NO induction. The IC50values of these cinnamic acid derivatives were between the IC50 of caffeic acid (226 μM) and the IC50 of chlorogenic acid (652 μM). Discussion This study clearly demonstrates that hydrophobic fraction A, butanolsoluble fraction B, and hydrophilic fraction C of the FLJ methanol extract suppressed IL-1β-induced NO production and iNOS expression in rat hepatocytes (Figure 2). Because the induction of NO and iNOS in IL-1β-stimulated hepatocytes mimics liver injury or inflammation [8,9], the suppression of NO/iNOS induction represents an anti-inflammatory effect. This bioassay system using hepatocytes revealed that chlorogenic acid, a constituent of major fraction C, strongly suppressed IL-1β-stimulated NO induction (Figures 4,5), suggesting that chlorogenic acid has anti-inflammatory effects. Given that the IC50 value of fraction C was 4046 μg/mL and the content of chlorogenic acid in fraction C was 8.09% (Table 2), the concentration of chlorogenic acid was assumed to be 924 μM (327 μg/mL). Given the IC50value of chlorogenic acid (652 μM), the NO suppression activity of fraction C can be almost entirely attributed to the activity of chlorogenic acid. In contrast, other low-MW constituents of fraction C, such as fructose, glucose, sucrose, and inositol, did not affect NO production (Table 2). Taken together, these findings indicate that chlorogenic acid is a major constituent of FLJ fraction C and inhibits the induction of NO production and iNOS expression in hepatocytes. This study established the ABC fractionation method, which is a procedure used in herbal medicine to purify and identify pharmacologically active constituents. The ABC fractionation method was useful to identify chlorogenic acid in the FLJ extract when combined with other methods, including HPLC and ultrafiltration. Problems caused by cytotoxic constituents present in the whole herbal extract may be avoided when this method is used. For example, although saponins, which are contained in several herbal medicines, may lyse hepatocytes, they were fractionated entirely into butanol-soluble fraction B by this method (unpublished data). Thus, this method may be preferable for the purification of active constituents from other herbal medicines. Chlorogenic acid inhibits the inducible expression of the iNOS gene, which contains two NF-κB binding sites [12]. Furthermore, chlorogenic acid suppressed the mRNA induction of several cytokines and chemokines that are involved in inflammation (Figure 5). There are also NF-κB binding sites in the promoters of all of the cytokine and chemokine genes examined in this study (data not shown), implying that the suppression of NO induction may be mediated by NF-κB. In LPS-treated mice, chlorogenic acid has prominent protective effects against liver injury by NF-κB [23], suggesting that chlorogenic acid has not only an anti-inflammatory effect but also a hepatoprotective effect. Recently, Lee et al. reported that the aqueous extract of Lonicera japonica flowers has an anti-inflammatory effect in LPS-administered rats and that pretreatment with the aqueous extract inhibits NF-κB Metabolites of chlorogenic acid (5-O-caffeoylquinic acid) are schematically shown. Chlorogenic acid is hydrolyzed to caffeic acid and (–)-quinic acid, and then metabolites of cinnamic acid derivatives (ferulic, isoferulic, and p-coumaric acids) are formed and circulate in human plasma [22,26]. Finally, sinapic and gallic acids are excreted into urine. The IC50values of the suppression of NO induction were determined using pure cinnamic acid derivatives in IL-1β-stimulated hepatocytes. The mean IC50values are indicated in parentheses. The results are representative of at least three independent experiments yielding similar data. Chemical structures were drawn with ChemDraw Std 12.0 (CambridgeSoft Corp., MA, USA).Nishizawa et al. HOAJ Biology 2012, activation and iNOS induction in the liver [24]. It is highly probable that hydrophilic chlorogenic acid is a major constituent of this aqueous extract. Furthermore, NF-κB is a key regulator in the induction of iNOS [9], and the Japanese Kampo medicine Inchinkoto and several drugs have been shown to influence the nuclear translocation of NF-κB and its binding to the iNOS gene promoter [7,12,25]. Therefore, these reports support our results that chlorogenic acid has an antiinflammatory effect by suppressing iNOS expression.The comparison of IC50 values among chlorogenic acid and structurally related compounds, i.e., cinnamic acid derivatives, revealed that caffeic acid is much more efficient than chlorogenic acid at suppressing NO induction (Table 2, Figure 7). Although cinnamic acid derivatives are the metabolites of chlorogenic acid and are present in human plasma, caffeic acid was not detected in fraction C of the FLJ extract (data not shown), and ferulic, isoferulic, and p-coumaric acids have not been isolated from Lonicera japonica [2]. Chlorogenic acid is absorbed in the intestine and metabolized in the liver and kidney to caffeic acid [22,26]. It is possible that caffeic acid is pharmacologically more active in the liver and plasma than chlorogenic acid. Therefore, it may be speculated that chlorogenic acid from FLJ has mild effects in humans by conversion to caffeic acid and other metabolites. Conclusions We analyzed the methanol extract of the flowers and buds of Lonicera japonica. Hydrophilic, butanol-soluble, and water-soluble fractions inhibited the induction of NO in IL-1β-stimulated hepatocytes, a simple in vitro liver injury model. Chlorogenic acid, a major constituent of the water-soluble fraction, and its metabolite caffeic acid also effectively suppressed the induction of NO production and iNOS expression. The anti-inflammatory activity of the water-soluble fraction of Lonicera japonica flowers and buds, as well as chlorogenic and caffeic acids, may have therapeutic potential for inflammatory diseases, including liver injury. List of abbreviations FLJ, Flos Lonicerae japonicae; NO, nitric oxide; IL, interleukin; iNOS, inducible nitric oxide synthase; LDH, lactose dehydrogenase; HPLC, high-performance liquid chromatography; IC50, half-maximal inhibitory concentration; SDS, sodium dodecyl sulfate; EF, elongation factor; RT, reverse transcription; PCR, polymerase chain reaction; SD, standard deviation; MW, molecular weight Author’s information 2Ritsumeikan Global Innovation Research Organization (R-GIRO), 3Research Organization of Science and Technology, 4Department of Surgery, Kansai Medical University, Hirakata, Osaka, Japan. 5Department of Food Sciences and Nutritional Health, Kyoto Prefectural University, Kyoto, Japan. 6Department of Pharmacy, College of Pharmaceutical Sciences, Ritsumeikan University, Kusatsu, Shiga, Japan. Authors’ contributions All authors read and approved the final manuscript. NO performed nitrite and LDH measurements and Western blot analyses. EY prepared hepatocytes and participated in the design and coordination of the study. T Okuyama prepared hepatocytes and carried out real-time RT-PCR. YY prepared hepatocytes and performed the nitrite measurements. T Okumura participated in the design and coordination of the study. KS analyzed sugar composition. YI participated in the design and coordination of the study and carried out purifications. MN conceived of the study, participated in its design and coordination, and drafted the manuscript. We wish to thank Ms. Noriko Kanazawa for her secretarial assistance and Ms. Yuna Takimoto for her technical assistance. This work was supported in part by research grants from Amino Up Chemical Co., Ltd. (Sapporo, Japan), Lion Corporation (Tokyo, Japan), and the R-GIRO of Ritsumeikan University. T Okumura, KS, YI, and MN received a research grant from the Amino Up Chemical Co., Ltd. (Sapporo, Japan). MN received a grant from the Lion Corporation (Kanagawa, Japan). Competing interests The Authors declare that they have no competing interests. Article history Received: 31-Jan-2012 Revised: 02-March-2012 Accepted: 15-Mar-2012 Published: 02-Apr-2012 References 1. Okuda T (ed.) (2008) Suikazura-ka Caprifoliaceae. In: Saishin Yakuyo Shokubutsugaku (The Latest Medicinal Plants). p. 186-187. Hirokawa Publishing Co., Tokyo, Japan (Japanese). 2. Shang X, Pan H, Li M, Miao X, Ding H: Lonicera japonica Thunb.: ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J Ethnopharmacol 2011; 138;(1.);1-21. 3. Clifford MN (1999) Chlorogenic acids and other cinnamates – nature, occurrence and dietary burden. J Sci Food Agric 79: 362-372. 4. Chen CY, Qi LW, Li HJ, Li P, Yi L, Ma HL, et al.: Simultaneous determination of iridoids, phenolic acids, flavonoids, and saponins in Flos Lonicerae and Flos Lonicerae Japonicae by HPLC-DAD-ELSD coupled with principal component analysis. J Sep Sci 2007; 30;(18.);3181-92. 5. Kang OH, Choi JG, Lee JH, Kwon DY: Luteolin isolated from the flowers of Lonicera japonica suppresses inflammatory mediator release by blocking NF-kappaB and MAPKs activation pathways in HMC-1 cells. Molecules 2010; 15;(1.);385-98. 6. Ryu KH, Rhee HI, Kim JH, Yoo H, Lee BY, Um KA, et al.: Anti-inflammatory and analgesic activities of SKLJI, a highly purified and injectable herbal extract of Lonicera japonica. Biosci Biotechnol Biochem 2010; 74;(10.);2022-8. 7. Matsuura T, Kaibori M, Araki Y, Matsumiya M, Yamamoto Y, Ikeya Y, et al.: Japanese herbal medicine, inchinkoto, inhibits inducible nitric oxide synthase induction in interleukin-1beta-stimulated hepatocytes. Hepatol Res 2012; 42;(1.);76-90. 8. Kitade H, Sakitani K, Inoue K, Masu Y, Kawada N, Hiramatsu Y, et al.: Interleukin 1 beta markedly stimulates nitric oxide formation in the absence of other cytokines or lipopolysaccharide in primary cultured rat hepatocytes but not in Kupffer cells. Hepatology 1996; 23;(4.);797-802. 9. Sakitani K, Nishizawa M, Inoue K, Masu Y, Okumura T, Ito S: Synergistic regulation of inducible nitric oxide synthase gene by CCAAT/enhancer-binding protein beta and nuclear factor-kappaB in hepatocytes. Genes Cells 1998; 3;(5.);321-30.10. Kanemaki T, Kitade H, Hiramatsu Y, Kamiyama Y, Okumura T: Stimulation of glycogen degradation by prostaglandin E2 in primary cultured rat hepatocytes. Prostaglandins 1993; 45;(5.);459-74. 11. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR: Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 1982; 126;(1.);131-8.Nishizawa et al. HOAJ Biology 2012, 12. Nakanishi H, Kaibori M, Teshima S, Yoshida H, Kwon AH, Kamiyama Y, et al.: Pirfenidone inhibits the induction of iNOS stimulated by interleukin-1beta at a step of NF-kappaB DNA binding in hepatocytes. J Hepatol 2004; 41;(5.);730-6. 13. Matsui K, Nishizawa M, Ozaki T, Kimura T, Hashimoto I, Yamada M, et al.: Natural antisense transcript stabilizes inducible nitric oxide synthase messenger RNA in rat hepatocytes. Hepatology 2008; 47;(2.);686-97. 14. Nishizawa M, Nakajima T, Yasuda K, Kanzaki H, Sasaguri Y, et al. (2000) Close kinship of human 20α-hydroxysteroid dehydrogenase gene with three aldo-ketoreductase genes. Genes Cells 5: 111-125. 15. Unezaki S, Nishizawa M, Okuda-Ashitaka E, Masu Y, Mukai M, Kobayashi S, et al.: Characterization of the isoforms of MOVO zinc finger protein, a mouse homologue of Drosophila Ovo, as transcription factors. Gene 2004; 336;(1.);47-58. 16. Park CM, Jin KS, Lee YW, Song YS: Luteolin and chicoric acid synergistically inhibited inflammatory responses via inactivation of PI3K-Akt pathway and impairment of NF-kappaB translocation in LPS stimulated RAW 264.7 cells. Eur J Pharmacol 2011; 660;(2-3.);454-9. 17. Nishizawa M, Okumura T, Ikeya Y, Kimura T: Regulation of inducible gene expression by natural antisense transcripts. Front Biosci 2012; 17;(938-58. 18. Hanada T, Yoshimura A: Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev 2002; 13;(4-5.);413-21 19. Tato CM, Cua DJ: Reconciling id, ego, and superego within interleukin-23. Immunol Rev 2008; 226;(103-11). 20. Liles WC, Van Voorhis WC: Review: nomenclature and biologic significance of cytokines involved in inflammation and the host immune response. J Infect Dis 1995; 172;(6.);1573-80. 21. Wasmuth HE, Tacke F, Trautwein C: Chemokines in liver inflammation and fibrosis. Semin Liver Dis 2010; 30;(3.);215-25. 22. Farah A, Monteiro M, Donangelo CM, Lafay S: Chlorogenic acids from green coffee extract are highly bioavailable in humans. J Nutr 2008; 138;(12.);2309-15. 23. Xu Y, Chen J, Yu X, Tao W, Jiang F, Yin Z, et al.: Protective effects of chlorogenic acid on acute hepatotoxicity induced by lipopolysaccharide in mice. Inflamm Res 2010; 59;(10.);871-7. 24. Lee JH, Ko WS, Kim YH, Kang HS, Kim HD, Choi BT: Anti-inflammatory effect of the aqueous extract from Lonicera japonica flower is related to inhibition of NF-kappaB activation through reducing I-kappaBalpha degradation in rat liver. Int J Mol Med 2001; 7;(1.);79-83. 25. Tanaka H, Uchida Y, Kaibori M, Hijikawa T, Ishizaki M, Yamada M, et al.: Na+/H+ exchanger inhibitor, FR183998, has protective effect in lethal acute liver failure and prevents iNOS induction in rats. J Hepatol 2008; 48;(2.);289-99. 26. Olthof MR, Hollman PC, Katan MB: Chlorogenic acid and caffeic acid are absorbed in humans. J Nutr 2001; 131;(1.);66-71. |







 Chlorogenic acid from the Japanese herbal medicine
Chlorogenic acid from the Japanese herbal medicine