| Ursolic Acid and Other Pentacyclic Triterpenoids: Anticancer Activities and Occurrence in Berries |
| 发布时间:2012-07-15 信息来源:admin 发布人:admin 点击次数:5347 |
Ursolic Acid and Other Pentacyclic Triterpenoids: Anticancer Activities and Occurrence in Berries Abstract :Ursolic acid and related pentacyclic triterpenoids have been isolated and identified as constituents of various berries, particularly cranberries (Vaccinium macrocarpon) and other Vaccinium species. In vitro studies have shown that these compounds possess anti-inflammatory and anti-cancer activities. These compounds reportedly inhibit the growth of numerous tumor cell lines including colon, breast, liver, prostate and leukemia and inhibit the expression and activity of cyclooxyge-nases. Among the berry triterpenoids, ursolic acid is the most studied. Ursolic acid has been found to induce apoptosis in tumor cells by activation of caspases and modulation of other pathways involved in cell proliferation and migration. These compounds may therefore play a complementary or synergistic role together with other berry constituents in chemoprevention. Further studies of bioavailability and in vivo activities are needed. Keywords :Ursolic acid · Triterpenoids · Anti-cancer · Cranberry · Blueberry · Vaccinium · Anti-inflammatory · Tumor · Apoptosis 1 Introduction Ursolic acid (3β-hydroxyurs-12-en-28-oic acid) is a pentacyclic triterpenoid that occurs in numerous plants and is a constituent of several herbal medicines marketed in Asia and worldwide for inflammatory conditions (Kim et al., 2004). As a potential functional food phytochemical, ursolic acid (UA) has received relatively scant attention, perhaps because little is known about its general distribution among edi-ble plants or its oral bioavailability. Numerous reports of UA’s in vitro activities against tumor cell lines have appeared in the literature (Novotny et al., 2001) and the possible mechanisms of action have been reviewed recently (Neto, 2007). The pentacyclic triterpenoids are a class of C30 isoprenoid compounds occurring widely in plants. Folding and cyclization of squalene leads to the dammarenyl ring system, which has a slightly different stereochemistry and ring structure from that of the major sterols (Dewick, 2001). Ring expansion and additional cyclization forms the characteristic fifth ring found in the lupeol, α-amyrin and β-amyrin skeletons (Fig. 2.1). Ursolic acid contains the β-amyrin skeleton; its C30 isomer α-amyrin is found in oleanolic acid. The 3-OH may be esterified with a phenolic acid. As these compounds are relatively nonpolar, they can be found in the waxy outer coating of fruits and other plant parts. Their role in the plant is not well understood. 2 Occurrence of Ursolic Acid and Other Triterpenoids in Vaccinium Berries Among berries, North American cranberry fruit (Vaccinium macrocarpon), in particular, contains a significant quantity of ursolic acid (Fig. 2.1a) in its peel. It is found in the aglycone form and as the cis and trans p-hydroxycinnamate esters (Murphy et al., 2003) shown in Fig. 2.1b, c. Quantitative analysis of cranberry fruit and products by liquid chromatography-mass spectrometry (LC-MS) found the ursolic acid content of whole cranberry fruit of different cultivars to range between 60 and 110 mg per 100 g of fresh fruit (Kondo, 2006). A similar content is found in sweetened, dried fruit. Considerably less ursolic acid is detected in jellied cran-berry sauce or commercial cranberry juice due presumably to its low solubility in water. A bioassay-guided fractionation approach was used to examine in vitro antitumor activities of whole cranberry fruit and juice, extracts and fractions, and finally,individual compounds or subfractions within structural classes. Initially, it was determined that an ethyl-acetate extract of whole cranberry fruit inhibited growth of human tumor cell lines in vitro (Yan et al., 2002). From ethyl-acetate soluble extracts we isolated and identified two hydroxycinnamate esters of ursolic acid that inhibited the growth of several types of tumor cells in vitro, includingMCF-7 breast, HT-29 colon, DU-145 prostate, H460 lung, ME180 cervical epidermoid, and K562 leukemia cell lines (Murphy et al., 2003). The concentration at which 50% growth inhibition occurred (GI50) ranged from 11 to 28 μg/mL for these esters depending on cell line. LC-MS analysis of various cranberry cultivars found that in addition to the parent ursolic acid, the hydroxycinnamate esters are present in whole cran-berry fruit in quantities averaging about 15–20 mg per 100 g of fresh fruit (Kondo,2006). Ursolic acid isolated from cranberry fruit was also reported to inhibit the proliferation of HepG2 human liver cancer cells (He and Liu, 2006). Other members of the genus Vaccinium have been found to contain ursolic acid.Highbush blueberries (Vaccinium corymbosum) also contain some ursolic acid in the peel. In a systematic study identifying the ethyl-acetate soluble constituents of highbush blueberry fruit, several sterols and triterpenoids, as well as phenolic acids, were isolated (Wang et al., 2000). Ursolic acid and its 19-hydroxy derivative,
3 Triterpenoids in Other Berries Sea buckthorn (Hippophae rhamnoides L.) is cultivated in the Baltic Sea region of northern Europe for use in food, fodder, pharmaceuticals and cosmetics, and has been investigated for its antiproliferative effects. A recent study examined the comparative activities of sea buckthorn berry extracts having varying compositions against cell proliferation in the Caco-2 (colon) and Hep G2 (liver) cancer cell lines (Grey et al., 2010). Among the extracts, the ethyl-acetate soluble extract showed the strongest antiproliferative effects on Caco-2 cells. Phytochemical analysis showed that ursolic acid was much higher in this extract than the others. Apoptosis was also detected based on an assay for histone-associated DNA fragments in the cyto-plasm. The antiproliferative activity of the ursolic-acid containing fraction was somewhat weaker in the HepG2 cell line, with polyphenolics-rich ethanol-soluble extract showing greater activity. The authors suggest that synergistic effects between the constituents are possible (Grey et al., 2010). Red berries of the decorative shrub winterberry (Ilex verticillata) were investigated for cytotoxic principles using a brine shrimp lethality assay (Fang and McLaughlin, 1989). Solvent partitioning resulted in a bioactive fraction from which ursolic acid was isolated as the active compound. However, winterberry is not recommended for human consumption (USDA/NRCS plant fact sheet at http://plants.usda.gov/factsheet/pdf/fs_ilve.pdf). Derivatives of ursolic and oleanolic acid have recently been reported as constituents of non-berry fruits including apples (Malus pumila), where the aglycones and several cinnamoyl and hydroxycinnamoyl esters were isolated from the peels (He and Liu, 2007). Anti-proliferative activities were measured in three tumor cell lines: HepG2 liver, MCF-7 breast and Caco-2 colon. Most of the triter-penoids exhibited EC50 values between 10 and 100 μM. The most effective inhibitors were 2α-hydroxyursolic acid and the coumaroyl esters of maslinic acid (2α-hydroxyoleanolic acid). 4 Anti-cancer and Anti-inflammatory Activities of Ursolic Acid and Its Esters The anti-proliferative activity of ursolic acid has been reported in a wide variety of cancer cell lines (Neto, 2007). Ursolic acid hydroxycinnamate esters isolated by us from cranberry fruit were evaluated for anti-tumor activity in a 60 tumor cell line panel through the National Cancer Institute’s Developmental Therapeutics program (http://dtp.nci.nih.gov/about.html). The esters inhibited the growth of several lung, colon, breast and renal cancers, melanoma and leukemia cell lines with GI50 val- ues based on sulforhodamine B (SRB) assay of between 1.2 and 11 μM (Kondo 2006). Ursolic acid may also affect migration and colony formation by cancer cells. We used clonogenic assays to assess effects of cranberry constituents on tumor colony formation over a 2 week period, showing that ursolic acid and cranberry proantho-cyanidins separately inhibited tumor colony formation in a dose-dependent manner in HT-29 and HCT116 colon tumor models (Liberty et al., 2009). In this study, the ursolic acid and cranberry proanthocyanidins both induced apoptosis, as detected by DNA fragmentation, but the effect varied with cell line. Both compounds caused a dose-dependent induction of apoptosis in HT-29 cells, whereas in the HCT116 cells, ursolic acid effectively induced apoptosis, but the proanthocyanidins had a weaker effect. Complementary effects of these compounds are likely to play a role in decreased tumor cell proliferation (Liberty et al., 2009). Most of the existing bioactivity studies on ursolic acid examine the activity of purified ursolic acid, either isolated from a plant or commercially obtained. The anti-inflammatory actions of pentacyclic triterpenoids are well known and structure-activity relationships have been reviewed (Safayhi and Sailer, 1997). For ursolic acid, several studies report anti-inflammatory activities in vivo, primarily observing reduced inflammation in mouse-ear edema models (Recio et al., 1995; Baricevic et al., 2001). The effects of ursolic acid on proinflammatory pathways observed in vitro include inhibition of COX-2 catalyzed prostaglandin biosynthe- sis (Ringbom et al., 1998). Ursolic acid inhibited COX-2 transcription in a human mammary oncogenic epithelial cell line (184B5/HER) and the observed suppres- sion of gene expression involved the protein kinase C signal transduction pathway (Subbaramaiah et al., 2000). Recently, cranberry extracts were evaluated for their anti-inflammatory activity, and a methanol-soluble extract was found to inhibit the activity of COX-2 at 50 μg/mL based on measuring conversion of arachidonic acid to prostaglandin E2 (Huang et al., 2009). The most active subfraction in this study, which inhibited COX-2 activity by 85% at a concentration of 10 μg/mL, was analyzed by LC-MS and found to contain ursolic acid and its hydroxycinnamate esters. The methanol fraction and active subfractions also inhibited the TNF-induced activation of NF-κB in Jurkat cells as well as NF-κB transcription in human T lymphocytes. Other possible anti-inflammatory mechanisms for ursolic acid include induction of NF-κB mediated expression of inducible nitric oxide synthase (iNOS) and TNF-α in macrophages, implying a possible anti-carcinogenic mechanism involving46 C.C. Neto enhanced NO production (You et al., 2001). In the same cranberry anti-inflammation study described above, cranberry extracts were also evaluated for iNOS activity in RAW 264.7 mouse macrophages. However, the extracts had no effect on iNOS activity at concentrations of 100 μg/mL or less (Huang et al., 2009). Despite its being recognized as an anti-inflammatory (Kim et al., 2004), ursolic acid has received relatively little attention as a functional food factor. Data on in vivo anti-cancer effects are quite scarce and most involve mouse paw edema models. One of the few in vivo anticancer studies of ursolic acid appeared in 2001. This mouse model study reported that a dose of 100 mg/kg inhibited murine fibrosarcoma FSaII growth (Lee et al., 2001). 5 Mechanisms of Action The in vitro anti-tumor activity of ursolic acid was reviewed in 2001 (Novotny et al., 2001). In addition to its anti-inflammatory activity, ursolic acid reduces the prolif- eration of many tumor cell lines and many possible mechanisms of action have been addressed. Studies in B16 cells and MCF-7 breast carcinoma cells showed an early G1 cytostatic effect for ursolic acid (Es-saady et al., 1996a, b). Enhancement of intracellular Ca2+ signaling is thought to play a role in reducing proliferation (Novotny et al., 2001). Ursolic acid’s ability to induce apoptosis in many different cell types is likely to play a major role in its anti-proliferative activity. In HT-29 colon cells, ursolic acid decreased proliferation by induction of apoptosis accompanied by activation of caspases-3, 8 and 9 (Andersson et al., 2003). Ursolic acid-induced apoptosis in HL-60 leukemia cells was observed to be mediated by intracellular Ca2+ release (Baek et al., 1997). In human prostate cells, apoptosis was accompanied by enhanced release of cytochrome c, caspase activation, and down-regulation of inhibitor of apoptosis proteins (c-IAPs) (Choi et al., 2000). Increased expression of p21WAF1,a gene regulated by p53 and thought to induce tumor suppression through inhibition of cyclin-dependent kinases (CDKs), has also been reported (Kim et al., 2000). In B16F-10 melanoma cells, ursolic acid at non-cytotoxic concentrations (10–50 μM) resulted in apoptosis accompanied by upregulation of the tumor suppressor gene p53 and caspase-3 and down-regulation of anti-apoptotic gene Bcl-2 (Manu and Kuttan, 2008). This decrease in NF-κB-mediated activation of Bcl-2 occurred with the inhibition of several transcription factors in the NF-κB pathway. A reduction was seen in the expression of pro-inflammatory cytokines IL-1β and IL-6, and TNF-α and GM-CSF. Caspase-3 activation by ursolic acid through the mitochon- drial pathway with upregulation of pro-apototic Bax and a decrease in Bcl-2 was reported in M4Beu human melanoma cells (Duval et al., 2008). Further, studies in an endometrial cancer cell line SNG-II showed that treatment with 50 μMursolic acid decreased activation of the phosphatidylinositol-3-kinase-Akt (PI3K-Akt) pathway and the mitogen activated protein kinase (MAPK) pathways, which are associated with the high level of Akt phosphorylation often seen in endometrial tumors (Achiwa et al., 2007) Ursolic acid has been shown to decrease the expression of matrix metalloproteinase-9 (MMP-9) (Cha et al., 1996) 31. Matrix metalloproteinase activities are involved in the remodeling of the extracellular matrix, part of the tumor micro-environment, and are thus linked to tumor invasion and increased risk of metastasis (Björklund and Koivunen, 2005). Ursolic acid has been shown to decrease cell viabililty and induce apoptosis in prostate cancer cells (Kassi et al., 2007). Since prostate cancers often metastasize, we have studied the effects of cran- berry constituents on matrix metalloproteinase expression in DU-145 prostate tumor cells. The hydroxycinnamoyl esters of ursolic acid were evaluated by us in a DU-145 prostate tumor model and were found to strongly inhibit expression of both MMP- 2 and MMP-9 at micromolar concentrations (Kondo et al., unpublished results). While polyphenolics from cranberry fruit also inhibited MMP expression (Neto et al., 2006), the triterpenoids were observed to do so at much lower concentrations. 6 Future Research Directions Considering the anti-proliferative activities of triterpenoids such as ursolic acid in cancer cells, the various molecular pathways affected, and the occurrence of these compounds in cranberries and other fruit, there is a clear need for further research on these compounds as potential functional food factors. Studies on their bioavailabil- ity and the nature of metabolites formed in vivo would be helpful in determining the role triterpenoids may play in preventing cancers and inflammatory diseases.The oral bioavailability of these compounds is not well-known and should be evaluated. Complementary or synergistic effects with other berry constituents such as flavonoids and carotenoids should be considered, as well as the effect of processing on the triterpenoid content in products derived from fruits. References Achiwa Y, Hasegawa K, Udagawa Y (2007) Regulation of the phosphatidylinositol 3-kinase-Akt and the mitogen-activated protein kinase pathways by ursolic acid in human endometrial cancer cells. Biosci Biotechnol Biochem 71:31–37 Andersson D, Liu JJ, Nilsson A, Duan RD (2003) Ursolic acid inhibits proliferation and stimulates apoptosis in HT29 cells following activation of alkaline sphingomyelinase. Anticancer Res 23:3317–3322 Baek JH, Lue YS, Kang CM, Kim JA, Kwon KS, Son HC, Kim KW (1997) Intracellular Ca2+ release mediates ursolic acid-induced apoptosis in human leukemic HL-60 cells. Int J Cancer 73:725–728 Baricevic D, Sosa S, Della Loggia R, Tubaro A, Simonovska B, Krasna A, Zupancic A (2001) Topical anti-inflammatory activity of Salvia officianalis L. leaves: the relevance of ursolic acid. J Ethnopharmacol 75:125–132 Björklund M, Koivunen E (2005). Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta 1755:37–69 Cha HJ, Bae SK, Lee HY, Lee O.H, Sato H, Setki M, Park BC, Kim KW (1996) Anti-invasive activity of ursolic acid correlates with the reduced expression of matrix metalloproteinase-9 (MMP-9) in HT1080 human fibrosarcoma cells. Cancer Res 56:2281–228448 C.C. Neto Choi YH, Baek JH, Yoo MA, Chung HY, Kim ND, Kim KW (2000) Induction of apoptosis by ursolic acid through activation of caspases and down-regulation of c-IAPs in human prostate epithelial cells. Int J Oncol 17:565–571 Dewick PM (2001) Medicinal natural products, a biosynthetic approach, 2nd edn. Wiley, Chichester, pp 212–219 Duval RE, Harmand PO, Jayat-Vignoles C, Cook-Moreau J, Pinon A, Delage C, Simon A (2008) Differential involvement of mitochondria during ursolic-acid induced apoptotic process in HaCaT and M4Beu cells. Oncol Rep 19:145–149 Es-saady D, Simon A, Jayat-Vignoles C, Chulia AJ, Delage C (1996a) MCF-7 cell cycle arrested at G1 through ursolic acid and increased reduction of tetrazolium salts. Anticancer Res 16: 481–486 Es-saady D, Simon A, Ollier M, Maurizis JC, Chulia AJ, Delage C (1996b) Inhibitory effect of ursolic acid on B16 proliferation through cell cycle arrest. Cancer Lett 106:193–197 Fang XP, McLaughlin JL (1989) Ursolic acid, a cytotoxic component of the berries of Ilex verticillata. Fitoterapia 61:176–177 Grey C, Widen C, Adlercreutz P, Rumpunen K, Duan RD (2010) Antiproliferative effects of sea buckthorn (Hippophae rhamnoides L.) extracts on human colon and liver cancer cell lines. Food Chem 120:1004–1010 He X, Liu RH (2006) Cranberry phytochemicals: isolation, structure elucidation and their anti- proliferative and antioxidant activities. J Agric Food Chem 54:7069–7074 He X, Liu RH (2007) Triterpenoids isolated from apple peels have potent anti-proliferative activ- ity and may be partially responsible for apple’s anticancer activity. J Agric Food Chem 55: 4366–4370 Huang Y, Nikolic D, Pendland S, Doyle BJ, Locklear TJ, Mahady GB (2009) Effects of cranberry extracts and ursolic acid derivatives on P-fimbriated Escherichia coli, COX-2 activity, proinflammatory cytokine release and the NF-κB transcriptional response in vitro. Pharm Biol 47:18–25 Kassi E, Papoutsi Z, Pratsinis H, Aligiannis N, Manoussakis M, Moutsatsou P (2007) Ursolic acid, a naturally occurring triterpenoid, demonstrates anticancer activity on human prostate cancer cells. J Cancer Res Clin Oncol 133:493–500 Kim DK, Baek JH, Kang CM, Yoo MA, Sung JW, Kim DK, Chung HY, Kim ND, Choi YH et al (2000) Apoptotic activity of ursolic acid may correlated with the inhibition of initiation of DNA replication. Int J Cancer 87:629–636 Kim KA, Lee JS, Park HJ, Kim JW, Kim CJ, Shim IS, Kim NJ, Han SM Lim S (2004) Inhibition of cytochrome P450 activities by oleanolic acid and ursolic acid in human liver microsomes. Life Sci 74:2769–2779 Kondo M (2006) Phytochemical studies of extracts from cranberry (Vaccinium macrocarpon) with anti-cancer, anti-fungal and cardioprotective properties. M.S. Thesis, University of Massachusetts Dartmouth Lee I, Lee J, Lee YH, Leonard J (2001) Ursolic-acid induced changes in tumor growth, O2 consumption, and tumor interstitial fluid pressure. Anticancer Res 21(4A): 2827–2833 Liberty AM, Amoroso JW, Hart PE, Neto CC (2009) Cranberry PACs and triterpenoids: anticancer activities in colon tumor cell lines. Proceedings of the second international symposium on human health effects of fruits and vegetables. Acta Hortic 841:61–66 Manu KA, Kuttan G (2008) Ursolic acid induces apoptosis by activating p53 and caspase-3 gene expressions and suppressing NF-kB mediated activation of bcl-2 in B16F-10 melanoma cells. Int Immunopharmacol 8:974–981 Murphy BT, MacKinnon SL, Yan X, Hammond GB, Vaisberg AJ, Neto CC (2003) Identification of triterpene hydroxycinnamates with in vitro anti-tumor activity from whole cranberry fruit (Vaccinium macrocarpon). J Agric Food Chem 51:3541–3545 Neto CC (2007) Cranberry and its phytochemicals: a review of in vitro anticancer studies. J Nutr 137:186S–193S Neto CC, Krueger CG, Lamoureaux TL, Kondo M, Vaisberg AJ, Hurta RAR, Curtis S, Matchett MD, Yeung H, Sweeney-Nixon MI, Reed JD (2006) MALDI-TOF MS characterization of proanthocyanidins from cranberry fruit (Vaccinium macrocarpon) that inhibit tumor cell growth and matrix metalloproteinase expression in vitro. J Sci Food Agric 86:18–25 Novotny L, Vachalkova A, Biggs D (2001) Ursolic acid: an anti-tumorigenic and chemopreventive activity minireview. Neoplasma 48:241–246 Ono M, Koto M, Komatsu H, Igoshi K, Kobayashi H, Ito Y, Nohara T (2004) Cytotoxic triterpenes and sterol from the fruit of rabbiteye blueberry (Vaccinium ashei). Food Sci Technol Res 10: 56–59 RecioMC, Giner RM,Manez S, Gueho J, Julien HR, Hostettmann K, Rios JL (1995) Investigations on the steroidal anti-inflammatory activity of triterpenoids from Diospyros leucomelas.Planta Med 61:9–12 Ringbom T, Segura L, Noreen Y, Perera P, Bohlin L (1998) Ursolic acid from Plantago major, a selective inhibitor of cyclooxygenase-2 catalyzed prostaglandin biosynthesis. J Nat Prod 61:1212–1215 Safayhi H, Sailer E-R (1997) Anti-Inflammatory actions of pentacyclic triterpenes. Planta Med 63:487–493 Subbaramaiah K, Michaluart P, Sporn MB, Dannenberg AJ (2000) Ursolic acid inhibits cyclooxygenase-2 transcription in human mammary epithelial cells. Cancer Res 60:2399–2404 Szakiel A, Mroczek A (2007) Distribution of triterpene acids and their derivatives in organs of cowberry (Vaccinium vits-idaea, L.) plant. Acta Biochim 54:733–740 Wang M, Li J, Shao Y, Huang T, Huang C, Chin C, Rosen RT, Ho CT (2000) Anti-oxidative and cytotoxic components of highbush blueberry (Vaccinium corymbosum L.), In: Phytochemicals and phytopharmaceuticals. AOCS Press, Champaign, IL, pp 271–277 Yan X, Murphy BT, Hammond GB, Vinson JA, Neto CC (2002) Antioxidant activities and anti- tumor screening of extracts from cranberry fruit (Vaccinium macrocarpon). J Agric Food Chem 20:5844–5849 You HJ, Choi CY, Kim JY, Park SJ, Hahm KS, Jeong HG (2001) Ursolic acid enhances nitric oxide and tumor necrosis factor-a production via nuclear factor-kB activation in the resting macrophages. FEBS Lett 509:156–160
|







 Ursolic Acid and Other Pentacyclic Triterpenoids: Anticancer Activities and Occurrence in Berries
Ursolic Acid and Other Pentacyclic Triterpenoids: Anticancer Activities and Occurrence in Berries 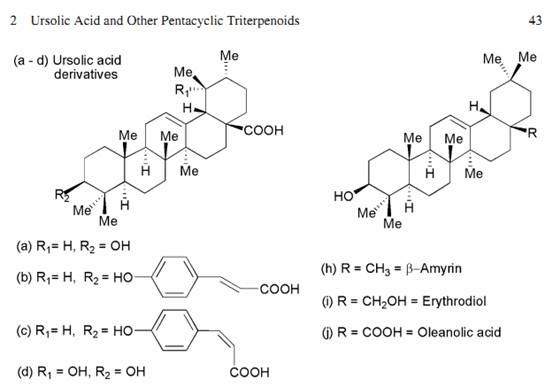

 pomolic acid (Fig. 2.1d), both isolated from blueberry fruit, were reported to inhibit proliferation and DNA synthesis in the HL-60 leukemia cell line at μM concentra- tions (Wang et al., 2000). A study of triterpenes and sterols isolated from the fruit44 C.C. Neto of “rabbiteye” blueberry (Vaccinium ashei) also found that ursolic acid, β-amyrin and a glucoside of the common sterol β-sitosterol (Fig. 2.1g) inhibited the growth of HCT 116 human colon cancer cells and PC-12 adrenal pheochromocytoma cells at micromolar concentrations (Ono et al., 2004). Other triterpenes identified in the fruit with lesser activity were α-amyrin (Fig. 2.1e), uvaol (Fig. 2.1f), erythrodiol (Fig. 2.1i), lupeol and betulin (Fig. 2.1k, l). Vaccinium vitis-idaea, known variously as cowberry, lingonberry and partridge berry, was reported to contain ursolic acid in fruits and leaves, with somewhat lesser amounts in the stems and rhizomes (Szakiel and Mroczek, 2007). Oleanolic acid (Fig. 2.1h), an isomer of ursolic acid differing only in the position of the C-29 methyl group, was also found in the plant. Both ursolic and oleanolic acid have been recognized as having anti-inflammatory properties (Safayhi and Sailer, 1997).
pomolic acid (Fig. 2.1d), both isolated from blueberry fruit, were reported to inhibit proliferation and DNA synthesis in the HL-60 leukemia cell line at μM concentra- tions (Wang et al., 2000). A study of triterpenes and sterols isolated from the fruit44 C.C. Neto of “rabbiteye” blueberry (Vaccinium ashei) also found that ursolic acid, β-amyrin and a glucoside of the common sterol β-sitosterol (Fig. 2.1g) inhibited the growth of HCT 116 human colon cancer cells and PC-12 adrenal pheochromocytoma cells at micromolar concentrations (Ono et al., 2004). Other triterpenes identified in the fruit with lesser activity were α-amyrin (Fig. 2.1e), uvaol (Fig. 2.1f), erythrodiol (Fig. 2.1i), lupeol and betulin (Fig. 2.1k, l). Vaccinium vitis-idaea, known variously as cowberry, lingonberry and partridge berry, was reported to contain ursolic acid in fruits and leaves, with somewhat lesser amounts in the stems and rhizomes (Szakiel and Mroczek, 2007). Oleanolic acid (Fig. 2.1h), an isomer of ursolic acid differing only in the position of the C-29 methyl group, was also found in the plant. Both ursolic and oleanolic acid have been recognized as having anti-inflammatory properties (Safayhi and Sailer, 1997).